Neuroprotection by glial metabotropic glutamate receptors is mediated by transforming growth factor-beta
- PMID: 9822720
- PMCID: PMC6793276
- DOI: 10.1523/JNEUROSCI.18-23-09594.1998
Neuroprotection by glial metabotropic glutamate receptors is mediated by transforming growth factor-beta
Abstract
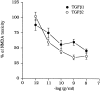
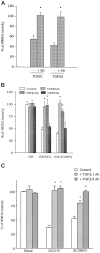
The medium collected from cultured astrocytes transiently exposed to the group-II metabotropic glutamate (mGlu) receptor agonists (2S,1'R, 2'R,3'R)-2-(2,3-dicarboxycyclopropyl)glycine (DCG-IV) or (S)-4-carboxy-3-hydroxyphenylglycine (4C3HPG) is neuroprotective when transferred to mixed cortical cultures challenged with NMDA (). The following data indicate that this particular form of neuroprotection is mediated by transforming growth factor-beta (TGFbeta). (1) TGFbeta1 and -beta2 were highly neuroprotective against NMDA toxicity, and their action was less than additive with that produced by the medium collected from astrocytes treated with DCG-IV or 4C3HPG (GM/DCG-IV or GM/4C3HPG); (2) antibodies that specifically neutralized the actions of TGFbeta1 or -beta2 prevented the neuroprotective activity of DCG-IV or 4C3HPG, as well as the activity of GM/DCG-IV or GM/4C3HPG; and (3) a transient exposure of cultured astrocytes to either DCG-IV or 4C3HPG led to a delayed increase in both intracellular and extracellular levels of TGFbeta. We therefore conclude that a transient activation of group-II mGlu receptors (presumably mGlu3 receptors) in astrocytes leads to an increased formation and release of TGFbeta, which in turn protects neighbor neurons against excitotoxic death. These results offer a new strategy for increasing the local production of neuroprotective factors in the CNS.
Figures




References
-
- Adams J, Collaco-Moraes Y, de Belleroche J. Cyclooxygenase-2 induction in cerebral cortex: an intracellular response to synaptic excitation. J Neurochem. 1996;66:6–13. - PubMed
-
- Bruno V, Wroblewska B, Wroblewska JT, Fiore L, Nicoletti F. Neuroprotective activity of N-acetylaspartylglutamate in cultured cortical cells. Neuroscience. 1998;3:751–757. - PubMed
-
- Buisson A, Choi DW. The inhibitory mGluR agonist, S-4-carboxy-3-hydroxyphenylglycine, selectively attenuates NMDA neurotoxicity and oxygen-glucose deprivation-induced neuronal death. Neuropharmacology. 1995;34:1081–1087. - PubMed
-
- Buisson A, Nicole O, Nouvelot A, MacKenzie ET, Vivien D. Reduction of NMDA-induced toxicity by transforming growth factor-β1. Soc Neurosci Abstr. 1997;23:897.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
