Morphology of single geniculocortical afferents and functional recovery of the visual cortex after reverse monocular deprivation in the kitten
- PMID: 9822746
- PMCID: PMC2452997
- DOI: 10.1523/JNEUROSCI.18-23-09896.1998
Morphology of single geniculocortical afferents and functional recovery of the visual cortex after reverse monocular deprivation in the kitten
Abstract
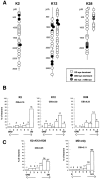
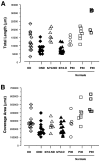
To investigate the possible anatomical basis for the functional recovery of visual cortical responses after reverse monocular deprivation, we have studied the morphology of single geniculocortical afferents to area 17. In kittens reverse-sutured for 10 d after an initial week of monocular deprivation, single-unit and intrinsic signal optical recordings confirmed that the effects of the initial deprivation were largely reversed. Responses through the originally nondeprived (OND) eye were drastically diminished, but remained much more selective for orientation than after an initial monocular deprivation (Crair et al., 1997). Responses through the originally deprived (OD) eye recovered completely. Geniculocortical afferent arbors in layer IV of area 17 were filled by iontophoresis of Phaseolus lectin into lamina A of the lateral geniculate nucleus (LGN) and were serially reconstructed. Arbors serving both the OD and the OND eye were analyzed. The plastic changes of both OD and OND arbors were evaluated by comparison with arbors reconstructed in normal animals and in animals studied after an equivalent initial period of deprivation (Antonini and Stryker, 1996). These analyses demonstrate that closure of the OND eye caused a substantial shrinkage of the arbors serving that eye. Moreover, reopening the OD eye induced regrowth only in some arbors, whereas others appeared to be largely unaffected and continued to have the characteristics of deprived arbors. Quantitatively, the initial and the second deprivation caused similar proportional changes in total arbor length and numbers of branches, whereas several other features were more severely affected by the initial deprivation.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
