Neurons containing hypocretin (orexin) project to multiple neuronal systems
- PMID: 9822755
- PMCID: PMC6793310
- DOI: 10.1523/JNEUROSCI.18-23-09996.1998
Neurons containing hypocretin (orexin) project to multiple neuronal systems
Abstract
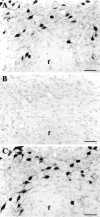
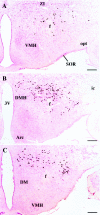
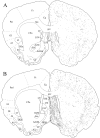
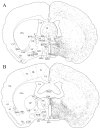
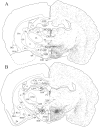
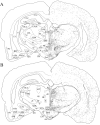
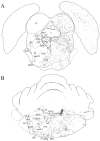
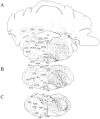
The novel neuropeptides called hypocretins (orexins) have recently been identified as being localized exclusively in cell bodies in a subregion of the tuberal part of the hypothalamus. The structure of the hypocretins, their accumulation in vesicles of axon terminals, and their excitatory effect on cultured hypothalamic neurons suggest that the hypocretins function in intercellular communication. To characterize these peptides further and to help understand what physiological functions they may serve, we undertook an immunohistochemical study to examine the distribution of preprohypocretin-immunoreactive neurons and fibers in the rat brain. Preprohypocretin-positive neurons were found in the perifornical nucleus and in the dorsal and lateral hypothalamic areas. These cells were distinct from those that express melanin-concentrating hormone. Although they represent a restricted group of cells, their projections were widely distributed in the brain. We observed labeled fibers throughout the hypothalamus. The densest extrahypothalamic projection was found in the locus coeruleus. Fibers were also seen in the septal nuclei, the bed nucleus of the stria terminalis, the paraventricular and reuniens nuclei of the thalamus, the zona incerta, the subthalamic nucleus, the central gray, the substantia nigra, the raphe nuclei, the parabrachial area, the medullary reticular formation, and the nucleus of the solitary tract. Less prominent projections were found in cortical regions, central and anterior amygdaloid nuclei, and the olfactory bulb. These results suggest that hypocretins are likely to have a role in physiological functions in addition to food intake such as regulation of blood pressure, the neuroendocrine system, body temperature, and the sleep-waking cycle.
Figures




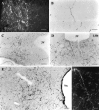
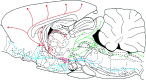
References
-
- Abercrombie M. Estimation of nuclear population from microtome sections. Anat Rec. 1946;94:239–247. - PubMed
-
- Akabayashi A, Wahlestedt C, Alexander JT, Leibowitz SF. Specific inhibition of endogenous neuropeptide Y synthesis in arcuate nucleus by antisense oligonucleotides suppresses feeding behavior and insulin secretion. Mol Brain Res. 1994;21:55–61. - PubMed
-
- Allen GV, Cechetto DF. Functional and anatomical organization of cardiovascular pressor and depressor sites in the lateral hypothalamic area: I. Descending projections. J Comp Neurol. 1992;315:313–332. - PubMed
-
- Allen GV, Cechetto DF. Functional and anatomical organization of cardiovascular pressor and depressor sites in the lateral hypothalamic area: II. Ascending projections. J Comp Neurol. 1993;330:421–438. - PubMed
-
- Bittencourt JC, Presse F, Arias C, Peto C, Vaughan J, Nahon JL, Vale W, Sawchenko PE. The melanin-concentrating hormone system of the rat brain: an immuno- and hybridization histochemical characterization. J Comp Neurol. 1992;319:218–245. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
