Postural hand synergies for tool use
- PMID: 9822764
- PMCID: PMC6793309
- DOI: 10.1523/JNEUROSCI.18-23-10105.1998
Postural hand synergies for tool use
Abstract
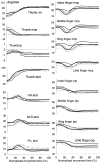
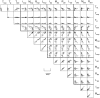
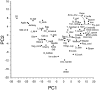
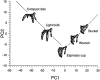
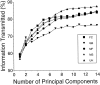
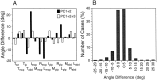
Subjects were asked to shape the right hand as if to grasp and use a large number of familiar objects. The chosen objects typically are held with a variety of grips, including "precision" and "power" grips. Static hand posture was measured by recording the angular position of 15 joint angles of the fingers and of the thumb. Although subjects adopted distinct hand shapes for the various objects, the joint angles of the digits did not vary independently. Principal components analysis showed that the first two components could account for >80% of the variance, implying a substantial reduction from the 15 degrees of freedom that were recorded. However, even though they were small, higher-order (more than three) principal components did not represent random variability but instead provided additional information about the object. These results suggest that the control of hand posture involves a few postural synergies, regulating the general shape of the hand, coupled with a finer control mechanism providing for small, subtle adjustments. Because the postural synergies did not coincide with grip taxonomies, the results suggest that hand posture may be regulated independently from the control of the contact forces that are used to grasp an object.
Figures





References
-
- Cutkosky MR, Howe RD. Human grasp choice and robotic grasp analysis. In: Venkataraman ST, Iberall T, editors. Dextrous robot hands. Springer; New York: 1990. pp. 5–31.
-
- Elliott JM, Connolly KJ. A classification of manipulative hand movements. Dev Med Child Neurol. 1984;26:283–296. - PubMed
-
- Flanders M, Herrmann U. Two components of muscle activation: scaling with the speed of arm movement. J Neurophysiol. 1992;67:913–943. - PubMed
-
- Glaser EM, Ruchkin DS. Principles of neurobiological signal analysis. Academic; New York: 1976.
-
- Gomez JE, Mason CR, Ebner TJ. Responses of precentral cortical neurons during whole hand grasping. Soc Neurosci Abstr. 1997;23:1555.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
