Ca2+ influx through carbachol-activated non-selective cation channels in guinea-pig gastric myocytes
- PMID: 9824715
- PMCID: PMC2231319
- DOI: 10.1111/j.1469-7793.1998.749ba.x
Ca2+ influx through carbachol-activated non-selective cation channels in guinea-pig gastric myocytes
Abstract
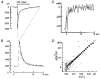
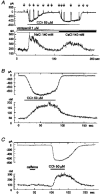
1. Ca2+ microfluorometry (100 microM K5 fura-2) and the voltage-clamp technique were combined to study the effect of carbachol (CCh, 50 microM) in inducing currents (ICCh) through non-selective cation channels (NSCCCh) and increments in global cytosolic Ca2+ concentration (Delta[Ca2+]c). 2. In Na+-containing bath solution, ICCh fell from an initial phasic to a subsequent small (5 %) tonic component; Delta[Ca2+]c fell to zero. Tonic ICCh and [Ca2+]c became prominent after substitution of extracellular 140 mM Na+ by 140 mM Cs+. Tonic ICCh and Delta[Ca2+]c were insensitive to intracellular heparin (3 mg ml-1) and ryanodine (4 microM), i.e. they did not depend on Ca2+ release from sarcoplasmic reticulum (SR). 3. Single channel currents of NSCCCh could be resolved in whole-cell recordings. Substitution of Na+ by Cs+ increased NSCCCh activity by one order of magnitude and slope conductance from 22 to 30 pS. Extracellular quinidine (3 microM) reversibly blocked the NSCCCh activity. 4. Both tonic ICCh and tonic Delta[Ca2+]c (a) followed a similar time course of activation, desensitization and facilitation, (b) were reversibly blocked by 3 microM quinidine, and (c) persisted upon block of SR Ca2+ release. 5. A Ca2+ fractional current of tonic ICCh (fCa) of 0.009 was calculated by comparing the ratio Delta[Ca2+]c (corrected for simultaneous Ca2+ redistribution) over ICCh with depolarization-induced *Delta[Ca2+]c (Delta[Ca2+]c calculated from ICa induced by a 400 ms depolarization from -60 to 0 mV at 2 mM [Ca2+]o, 145 mM [Cs+]o) over ICa. fCa was 0.023 at [Ca2+]o = 4 mM. 6. With 110 mM extracellular CaCl2 and 145 mM intracellular CsCl, ICCh reversed at +19.5 mV suggesting a permeability ratio PCa/PCs of 2.8. 7. We conclude that Ca2+ influx through NSCCCh under physiological [Ca2+]o could induce Delta[Ca2+]c. The fCa was, however, much smaller than the one calculated from the reversal potential.
Figures





Similar articles
-
Protein kinase C mediates the desensitization of CCh-activated nonselective cationic current in guinea-pig gastric myocytes.Pflugers Arch. 1998 Jun;436(1):1-8. doi: 10.1007/s004240050597. Pflugers Arch. 1998. PMID: 9560440
-
Efficacy of peak Ca2+ currents (ICa) as trigger of sarcoplasmic reticulum Ca2+ release in myocytes from the guinea-pig coronary artery.J Physiol. 1995 Apr 15;484 ( Pt 2)(Pt 2):287-306. doi: 10.1113/jphysiol.1995.sp020665. J Physiol. 1995. PMID: 7541467 Free PMC article.
-
Quinidine blockade of the carbachol-activated nonselective cationic current in guinea-pig gastric myocytes.Br J Pharmacol. 1995 Aug;115(8):1407-14. doi: 10.1111/j.1476-5381.1995.tb16631.x. Br J Pharmacol. 1995. PMID: 8564199 Free PMC article.
-
Nonselective cation channels activated by the stimulation of muscarinic receptors in mammalian gastric smooth muscle.J Smooth Muscle Res. 2003 Dec;39(6):231-47. doi: 10.1540/jsmr.39.231. J Smooth Muscle Res. 2003. PMID: 15048016 Review.
-
Voltage-dependent conductances of solitary ganglion cells dissociated from the rat retina.J Physiol. 1987 Apr;385:361-91. doi: 10.1113/jphysiol.1987.sp016497. J Physiol. 1987. PMID: 2443669 Free PMC article. Review.
Cited by
-
Calcium permeability of transient receptor potential canonical (TRPC) 4 channels measured by TRPC4-GCaMP6s.Korean J Physiol Pharmacol. 2017 Jan;21(1):133-140. doi: 10.4196/kjpp.2017.21.1.133. Epub 2016 Dec 21. Korean J Physiol Pharmacol. 2017. PMID: 28066150 Free PMC article.
-
Three distinct muscarinic signalling pathways for cationic channel activation in mouse gut smooth muscle cells.J Physiol. 2007 Jul 1;582(Pt 1):41-61. doi: 10.1113/jphysiol.2007.133165. Epub 2007 Apr 26. J Physiol. 2007. PMID: 17463038 Free PMC article.
-
Hyposmotic membrane stretch potentiated muscarinic receptor agonist-induced depolarization of membrane potential in guinea-pig gastric myocytes.World J Gastroenterol. 2002 Aug;8(4):724-7. doi: 10.3748/wjg.v8.i4.724. World J Gastroenterol. 2002. PMID: 12174386 Free PMC article.
-
Voltage-dependent inhibition of the muscarinic cationic current in guinea-pig ileal cells by SK&F 96365.Br J Pharmacol. 2000 Feb;129(4):695-702. doi: 10.1038/sj.bjp.0703115. Br J Pharmacol. 2000. PMID: 10683194 Free PMC article.
-
The developing relationship between receptor-operated and store-operated calcium channels in smooth muscle.Br J Pharmacol. 2002 Jan;135(1):1-13. doi: 10.1038/sj.bjp.0704468. Br J Pharmacol. 2002. PMID: 11786473 Free PMC article. Review.
References
-
- Aidley DJ, Stanfield PR. Permeability and selectivity. In: Aidley DJ, Stanfield PR, editors. Ion Channels, Molecules in Action. Cambridge: Cambridge University Press; 1996. pp. 121–160.
-
- Benham DF, Tsien RW. A novel receptor-operated Ca2+-permeable channel activated by ATP in smooth muscle. Nature. 1987;328:275–278. - PubMed
-
- Cole WC, Carl A, Sanders KM. Muscarinic suppression of Ca2+-dependent K current in colonic smooth muscle. American Journal of Physiology. 1989;257:C481–487. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

