Presynaptic inhibition by dopamine of a discrete component of GABA release in rat substantia nigra pars reticulata
- PMID: 9824719
- PMCID: PMC2231314
- DOI: 10.1111/j.1469-7793.1998.805ba.x
Presynaptic inhibition by dopamine of a discrete component of GABA release in rat substantia nigra pars reticulata
Abstract
1. Whole-cell patch clamp recordings were made from substantia nigra pars reticulata (SNr) neurones in rat midbrain slices. Monosynaptic IPSCs were evoked by electrical stimulation of the cerebral peduncle in the presence of the glutamate receptor antagonists CNQX (6-cyano-7-nitroquinoxaline-2,3-dione) and AP5 (2-amino-5-phosphonopentanoic acid). 2. IPSCs were predominantly outward at -70 mV (in 124/135 cells), with a reversal potential of -83 mV, a time to peak of 2.6 ms and a decay time constant of 6.5 ms. Faster inward IPSCs were also observed in thirty-five cells, with a time to peak of 1.0 ms, a decay time constant of 2.3 ms, and a reversal potential of -61 mV. Both IPSCs were sensitive to the GABAA receptor antagonists picrotoxin or bicuculline. 3. In cells recorded with Cs+-filled pipettes, the outward IPSC reversal potential was shifted to -76 mV, closer to the estimated Cl- equilibrium potential of -56 mV, while that of the inward IPSC was unchanged at -64 mV. 4. The outward IPSC was reversibly depressed by up to 100 % by dopamine in a concentration-dependent manner with an IC50 of 10.5 microM, while the inward IPSC was relatively insensitive. 5. Dopamine was without effect on cell holding current, or on outward IPSC reversal potential, but it increased paired-pulse IPSC facilitation, consistent with a presynaptic site of action. 6. The D1-like dopamine receptor agonist SKF 38393 (10 microM) depressed the outward IPSC by 43 %, while the D2-like dopamine receptor agonist quinpirole (10 microM) was without effect. 7. It is concluded that GABA-ergic synaptic input onto distal rather than proximal regions of SNr neurones is susceptible to presynaptic inhibition via a D1-like receptor. These inputs are probably from striato-nigral fibres, and their inhibition by dopamine is likely to influence the patterning of basal ganglia output.
Figures





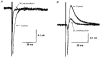
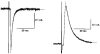



Similar articles
-
Intrinsic and integrative properties of substantia nigra pars reticulata neurons.Neuroscience. 2011 Dec 15;198:69-94. doi: 10.1016/j.neuroscience.2011.07.061. Epub 2011 Aug 2. Neuroscience. 2011. PMID: 21839148 Free PMC article. Review.
-
Electrophysiological investigation of adenosine trisphosphate-sensitive potassium channels in the rat substantia nigra pars reticulata.Neuroscience. 1996 Sep;74(2):499-509. doi: 10.1016/0306-4522(96)00151-0. Neuroscience. 1996. PMID: 8865200
-
Hippocampal CA1 lacunosum-moleculare interneurons: modulation of monosynaptic GABAergic IPSCs by presynaptic GABAB receptors.J Neurophysiol. 1995 Nov;74(5):2126-37. doi: 10.1152/jn.1995.74.5.2126. J Neurophysiol. 1995. PMID: 8592201
-
Dopamine D2 receptor mediated presynaptic inhibition of striatopallidal GABA(A) IPSCs in vitro.Neuropharmacology. 2001 Jul;41(1):62-71. doi: 10.1016/s0028-3908(01)00038-7. Neuropharmacology. 2001. PMID: 11445186
-
Dopamine D1 receptors facilitate GABAA synaptic currents in the rat substantia nigra pars reticulata.J Neurosci. 1998 Mar 15;18(6):2009-16. doi: 10.1523/JNEUROSCI.18-06-02009.1998. J Neurosci. 1998. PMID: 9482788 Free PMC article. Review.
Cited by
-
Extrastriatal dopaminergic circuits of the Basal Ganglia.Front Neuroanat. 2010 Oct 27;4:139. doi: 10.3389/fnana.2010.00139. eCollection 2010. Front Neuroanat. 2010. PMID: 21103009 Free PMC article.
-
Intrinsic and integrative properties of substantia nigra pars reticulata neurons.Neuroscience. 2011 Dec 15;198:69-94. doi: 10.1016/j.neuroscience.2011.07.061. Epub 2011 Aug 2. Neuroscience. 2011. PMID: 21839148 Free PMC article. Review.
-
Dopamine release in the basal ganglia.Neuroscience. 2011 Dec 15;198:112-37. doi: 10.1016/j.neuroscience.2011.08.066. Epub 2011 Sep 14. Neuroscience. 2011. PMID: 21939738 Free PMC article. Review.
-
Dopamine D1 Receptor Immunoreactivity on Fine Processes of GFAP-Positive Astrocytes in the Substantia Nigra Pars Reticulata of Adult Mouse.Front Neuroanat. 2017 Feb 1;11:3. doi: 10.3389/fnana.2017.00003. eCollection 2017. Front Neuroanat. 2017. PMID: 28203148 Free PMC article.
-
Insulin in the ventral tegmental area reduces hedonic feeding and suppresses dopamine concentration via increased reuptake.Eur J Neurosci. 2012 Aug;36(3):2336-46. doi: 10.1111/j.1460-9568.2012.08168.x. Epub 2012 Jun 20. Eur J Neurosci. 2012. PMID: 22712725 Free PMC article.
References
-
- Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends in Neurosciences. 1990;13:266–271. 10.1016/0166-2236(90)90107-L. - DOI - PubMed
-
- Bevan MD, Bolam JP, Crossman AR. Convergent synaptic input from the neostriatum and the subthalamus onto identified nigrothalamic neurons in the rat. European Journal of Neuroscience. 1994;6:320–334. - PubMed
-
- Cameron DL, Williams JT. Dopamine D1 receptors facilitate transmitter release. Nature. 1993;366:344–347. - PubMed
-
- Della Corte L, Bolam JP, Clarke DJ, Parry DM, Smith AD. Sites of [3H]taurine uptake in the rat substantia nigra in relation to the release of taurine from the striatonigral pathway. European Journal of Neuroscience. 1990;2:50–61. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

