Apamin- and nitric oxide-sensitive biphasic non-adrenergic non-cholinergic inhibitory junction potentials in the rat anococcygeus muscle
- PMID: 9824721
- PMCID: PMC2231329
- DOI: 10.1111/j.1469-7793.1998.835ba.x
Apamin- and nitric oxide-sensitive biphasic non-adrenergic non-cholinergic inhibitory junction potentials in the rat anococcygeus muscle
Abstract
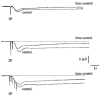
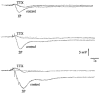
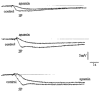
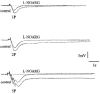
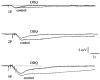
1. Changes in membrane potential following electrical field stimulation (EFS; 1, 2 and 5 pulses at 5 Hz, 0.5 ms duration, 60-80 V) of non-adrenergic non-cholinergic (NANC) inhibitory nerves in the rat isolated anococcygeus muscle were measured using standard intracellular recording techniques. Resting membrane potential ranged between -60 and -70 mV. 2. In the presence of guanethidine (30 microM), atropine (1 microM), propranolol (1 microM) and phentolamine (0.05 microM) to establish NANC conditions, the membrane potential depolarized to between -40 and -50 mV. Under these conditions, EFS caused pulse-dependent, tetrodotoxin (1 microM)-sensitive biphasic inhibitory junction potentials (IJPs) comprising a fast onset and time-to-peak phase followed by a second, slower phase that delayed repolarization. The duration of NANC IJPs ranged between 10 and 20 s. 3. Inhibition of small-conductance Ca2+-activated K+ channels with apamin (0.1 microM) selectively blocked the first fast phase of the NANC IJP, whereas inhibitors of large-conductance Ca2+-activated K+ channels (charybdotoxin and iberiotoxin) and ATP-sensitive K+ channels (glibenclamide) all had no effect on NANC IJPs. 4. Both the nitric oxide synthase inhibitor N G-nitro-L-arginine (L-NOARG; 100 microM) and the inhibitor of soluble guanylate cyclase 1-H-oxodiazol-[1,2,4]-[4,3-a] quinoxaline-1-one (ODQ; 10 microM) had no effect on the first fast phase of the NANC IJP. Each treatment, however, markedly inhibited the slow phase with the duration of the IJP reduced to between 1 and 3 s. The L-NOARG-resistant fast phase of the NANC IJP was almost abolished by the subsequent addition of apamin (0.1 microM). 5. In conclusion, the present study demonstrates unequivocal NANC nerve-mediated biphasic IJPs in the rat isolated anococcygeus. We propose that nitric oxide (NO), via activation of cGMP-dependent K+ channels, and a non-NO inhibitory factor which activates apamin-sensitive K+ channels contribute to NANC nerve-evoked IJPs in the rat anococcygeus.
Figures

Similar articles
-
NANC inhibitory neuromuscular transmission in the hamster distal colon.Pharmacol Res. 2006 Dec;54(6):452-60. doi: 10.1016/j.phrs.2006.09.004. Epub 2006 Sep 17. Pharmacol Res. 2006. PMID: 17035041
-
Characterization of the apamin- and L-nitroarginine-resistant NANC inhibitory transmission to the circular muscle of guinea-pig colon.J Auton Pharmacol. 1996 Jun;16(3):131-45. J Auton Pharmacol. 1996. PMID: 8884460
-
Neuronal mediators of inhibitory junction potentials and relaxation in the guinea-pig internal anal sphincter.J Physiol. 1996 Jun 1;493 ( Pt 2)(Pt 2):517-27. doi: 10.1113/jphysiol.1996.sp021400. J Physiol. 1996. PMID: 8782113 Free PMC article.
-
[The control of smooth muscle tissues by nonadrenergic noncholinergic (NANC) nerve fibres in the autonomic nervous system].J Smooth Muscle Res. 1995 Jun;31(3):67-78. doi: 10.1540/jsmr.31.67. J Smooth Muscle Res. 1995. PMID: 8563058 Review. Japanese.
-
Non-adrenergic non-cholinergic (NANC) transmission to smooth muscle: 35 years on.Prog Neurobiol. 1997 Jun;52(3):159-95. doi: 10.1016/s0301-0082(97)00012-9. Prog Neurobiol. 1997. PMID: 9234452 Review.
References
-
- Bennett MA. Non-adrenergic non-cholinergic (NANC) transmission to smooth muscle: 35 years on. Progress in Neurobiology. 1997;52:159–195. - PubMed
-
- Bolotina VM, Najibi S, Palacino JJ, Pagano PJ, Cohen RA. Nitric oxide directly activates calcium-dependent potassium channels in vascular smooth muscle. Nature. 1994;368:850–853. - PubMed
-
- Burnstock G. Purinergic nerves. Pharmacological Reviews. 1972;24:509–581. - PubMed
-
- Burnstock G. Overview: purinergic mechanisms. Annals of the New York Academy of Sciences. 1990;603:1–18. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

