The lipopolysaccharide of Bordetella bronchiseptica acts as a protective shield against antimicrobial peptides
- PMID: 9826332
- PMCID: PMC108708
- DOI: 10.1128/IAI.66.12.5607-5612.1998
The lipopolysaccharide of Bordetella bronchiseptica acts as a protective shield against antimicrobial peptides
Abstract
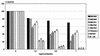
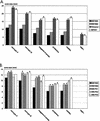
Resistance profiles of the two Bordetella species B. bronchiseptica and B. pertussis against various antimicrobial peptides were determined in liquid survival and agar diffusion assays. B. bronchiseptica exhibited significantly higher resistance against all tested peptides than B. pertussis. The most powerful agents acting on B. bronchiseptica were, in the order of their killing efficiencies, cecropin P > cecropin B > magainin-II-amide > protamine > melittin. Interestingly, for B. bronchiseptica, the resistance level was significantly affected by phase variation, as a bvgS deletion derivative showed an increased sensitivity to these peptides. Tn5-induced protamine-sensitive B. bronchiseptica mutants, which were found to be very susceptible to most of the cationic peptides, were isolated. In two of these mutants, the genetic loci inactivated by transposon insertion were identified as containing genes highly homologous to the wlbA and wlbL genes of B. pertussis that are involved in the biosynthesis of lipopolysaccharide (LPS). In agreement with this finding, the two peptide-sensitive mutants revealed structural changes in the LPS, resulting in the loss of the O-specific side chains and the prevalence of the LPS core structure. This demonstrates that LPS plays a major role in the resistance of B. bronchiseptica against the action of antimicrobial peptides and suggests that B. pertussis is much more susceptible to these peptides due to the lack of the highly charged O-specific sugar side chains.
Figures
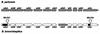

References
-
- Allen A G, Maskell D J. The identification, cloning and mutagenesis of a genetic locus required for lipopolysaccharide biosynthesis in Bordetella pertussis. Mol Microbiol. 1996;19:37–52. - PubMed
-
- Allen A G, Thomas R M, Cadisch J T, Maskell D J. Molecular and functional analysis of the lipopolysaccharide biosynthesis locus wlb from Bordetella pertussis, Bordetella parapertussis and Bordetella bronchiseptica. Mol Microbiol. 1998;29:27–38. - PubMed
-
- Amano K-I, Fukushi K, Watanabe M. Biochemical and immunological comparison of lipopolysaccharides from Bordetella species. J Gen Microbiol. 1990;136:481–487. - PubMed
-
- Aspedon A, Groisman E A. The antibacterial action of protamine: evidence for disruption of cytoplasmic membrane energization in Salmonella typhimurium. Microbiology. 1996;142:3389–3397. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

