The type IV leader peptidase/N-methyltransferase of Vibrio vulnificus controls factors required for adherence to HEp-2 cells and virulence in iron-overloaded mice
- PMID: 9826339
- PMCID: PMC108715
- DOI: 10.1128/IAI.66.12.5659-5668.1998
The type IV leader peptidase/N-methyltransferase of Vibrio vulnificus controls factors required for adherence to HEp-2 cells and virulence in iron-overloaded mice
Abstract
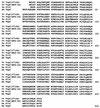
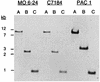
Vibrio vulnificus expresses a number of potential virulence determinants that may contribute to its ability to cause a severe and rapidly disseminating septicemia in susceptible hosts. We have cloned and characterized two genes encoding products related to components of the type IV pilus biogenesis and general secretory (type II) pathways by complementation of a type IV peptidase/N-methyltransferase (PilD) mutant of Pseudomonas aeruginosa with a V. vulnificus genomic library. One of the genes (vvpD) encodes a protein homologous to PilD and other members of the type IV peptidase family that completely restores this activity in a P. aeruginosa mutant deficient in the expression of PilD. The other gene (vvpC) encodes a homolog of PilC from P. aeruginosa, where it is essential for assembly of type IV pili. Phenotypic characterization of a V. vulnificus vvpD mutant, constructed by allelic exchange, showed that VvpD is required for the expression of surface pili, suggesting that the pili observed on V. vulnificus are of the type IV class. This mutant was also unable to secrete at least three extracellular degradative enzymes, and the localization of one of these (the cytolysin/hemolysin) to the periplasmic space indicates that these proteins are normally exported via the type II secretion pathway. Loss of VvpD resulted in significant decreases in CHO cell cytotoxicity, adherence to HEp-2 cells, and virulence in a mouse model. Capsule formation and serum resistance were not affected in the vvpD mutant, indicating that in addition to capsule, virulence of V. vulnificus requires type IV pili and/or extracellular secretion of several exoenzymes.
Figures


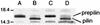


References
-
- Alm R A, Mattick J S. Genes involved in the biogenesis and function of type-4 fimbriae in Pseudomonas aeruginosa. Gene. 1997;192:89–98. - PubMed
-
- Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. - PubMed
-
- Anonymous. Vibrio vulnificus infections associated with raw oyster consumption—Florida, 1981–1992. Morbid Mortal Weekly Rep. 1993;42:405–407. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources

