Systemic and mucosal immune responses after intranasal administration of recombinant Mycobacterium bovis bacillus Calmette-Guérin expressing glutathione S-transferase from Schistosoma haematobium
- PMID: 9826340
- PMCID: PMC108716
- DOI: 10.1128/IAI.66.12.5669-5676.1998
Systemic and mucosal immune responses after intranasal administration of recombinant Mycobacterium bovis bacillus Calmette-Guérin expressing glutathione S-transferase from Schistosoma haematobium
Abstract
A major goal of current vaccine development is the induction of strong immune responses against protective antigens delivered by mucosal routes. One of the most promising approaches in that respect relies on the use of live recombinant vaccine carriers. In this study, Mycobacterium bovis BCG was engineered to produce an intracellular glutathione S-transferase from Schistosoma haematobium (Sh28GST). The gene encoding Sh28GST was placed under the control of the mycobacterial hsp60 promoter on a replicative shuttle plasmid containing a mercury resistance operon as the only selectable marker. The recombinant Sh28GST produced in BCG bound glutathione and expressed enzymatic activity, indicating that its active site was properly folded. Both intraperitoneal and intranasal immunizations of BALB/c mice with the recombinant BCG resulted in strong anti-Sh28GST antibody responses, which were enhanced by a boost. Mice immunized intranasally produced a mixed response with the production of Sh28GST-specific immunoglobulin G1 (IgG1), IgG2a, IgG2b, and IgA in the serum. In addition, high levels of anti-Sh28GST IgA were also found in the bronchoalveolar lavage fluids, demonstrating that intranasal delivery of the recombinant BCG was able to induce long-lasting secretory and systemic immune responses to antigens expressed intracellularly. Surprisingly, intranasal immunization with the BCG producing the Sh28GST induced a much stronger specific humoral response than intranasal immunization with BCG producing the glutathione S-transferase from Schistosoma mansoni, although the two antigens have over 90% identity. This difference was not observed after intraperitoneal administration.
Figures

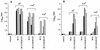




References
-
- Barclay W R, Busey W M, Dalgard D W, Good R C, Janicki B W, Kasik J E, Ribi E, Ulrich C E, Wolinsky E. Protection of monkeys against airborne tuberculosis by aerosol vaccination with Bacillus Calmette-Guérin. Am Rev Respir Dis. 1973;107:351–358. - PubMed
-
- Baulard A, Escuyer V, Haddad N, Kremer L, Locht C, Berche P. Mercury resistance as a selective marker for recombinant mycobacteria. Microbiology. 1994;141:1045–1050. - PubMed
-
- Baulard A, Kremer L, Supply P, Vidaud D, Bidart J M, Bellet D, Locht C. A new series of mycobacterial expression vectors for the development of live recombinant vaccines. Gene. 1996;176:149–154. - PubMed
-
- Colditz G A, Brewer T F, Berkey C S, Wilson M E, Burdick E, Fineberg H V, Mosteller F. Efficacy of BCG vaccine in the prevention of tuberculosis: meta-analysis of the published literature. JAMA. 1994;271:698–702. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

