Detection of anionic antimicrobial peptides in ovine bronchoalveolar lavage fluid and respiratory epithelium
- PMID: 9826377
- PMCID: PMC108753
- DOI: 10.1128/IAI.66.12.5948-5954.1998
Detection of anionic antimicrobial peptides in ovine bronchoalveolar lavage fluid and respiratory epithelium
Abstract
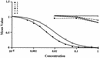
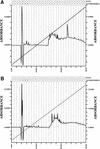
Three small antimicrobial anionic peptides (AP) were originally isolated from an ovine pulmonary surfactant. However, their presence in bronchoalveolar lavage (BAL) fluid and tissues of the respiratory tract is unknown. In this study, we made affinity-purified rabbit polyclonal and mouse monoclonal antibodies to synthetic H-DDDDDDD-OH. Antibody specificity was assessed by a competitive enzyme-linked immunosorbent assay (ELISA), and the exact epitope binding sites were determined with analog peptides synthesized on derivatized cellulose. These antibodies were used to detect AP in BAL fluid by ELISA and in respiratory tissues by Western blot analysis and immunocytochemistry. BAL fluid from 25 sheep contained 0.83 +/- 0.33 mM AP (mean +/- standard deviation; range, 0.10 to 1.59 mM) and was antimicrobial. The presence of AP in BAL fluid was confirmed by reverse-phase high-pressure liquid chromatography fractionation followed by matrix-assisted laser desorption/ionization (MALDI) mass spectrometry on those fractions which were positive by competitive ELISA and demonstrated antimicrobial activity. In Western blots, polyclonal antibody PAB96-1 and monoclonal antibody 1G9-1C2 (5.0 micrograms/ml) detected four bands in solubilized turbinate and tracheal epithelial cells (53.7, 31.2, 28.0, and 25.7 kDa) and five bands in lung homogenates (53.5, 37.1, 31.2, 28.0, and 25.7 kDa). Only a single band was seen in solubilized liver and small-intestine homogenates, and no bands were seen in blots containing BAL fluid, albumin, or kidney or spleen homogenates. In pulmonary-tissue sections, both antibodies PAB96-1 and 1G9-1C2 identified accumulated protein in the apical cytoplasm of the bronchial and bronchiolar epithelia, in the cytoplasm of pulmonary endothelial cells, and in an occasional alveolar macrophage. As a first step in identifying a candidate AP precursor gene(s), degenerate oligonucleotides representing all possible coding combinations for H-GADDDDD-OH and H-DDDDDDD-OH were synthesized and used to probe Southern blots of sheep genomic DNA. Following low-stringency washes and a 2-day exposure, strongly hybridizing bands could be identified. One degenerate oligonucleotide, SH87, was used as a hybridization probe to screen a sheep phage genomic library. Two independent phage contained the H-GADDDDD-OH coding sequence as part of a larger predicted protein. AP may originate as part of an intracellular precursor protein, with multistep processing leading to the release of the heptapeptide into mucosal secretions. There it may interact with other innate pulmonary defenses to prevent microbial infection.
Figures




References
-
- Bartlett J G. Bacteriological diagnosis of pulmonary infections. In: Sackner M A, editor. Diagnostic techniques in pulmonary disease. Vol. 16. New York, N.Y: Marcel Dekker, Inc.; 1981. pp. 707–745.
-
- Bligh E G, Dyer W J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. - PubMed
-
- Boman H G. Peptide antibiotics and their role in innate immunity. Annu Rev Immunol. 1995;13:61–92. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

