Evidence for multiple pathologic and protective mechanisms of murine cerebral malaria
- PMID: 9826380
- PMCID: PMC108756
- DOI: 10.1128/IAI.66.12.5972-5979.1998
Evidence for multiple pathologic and protective mechanisms of murine cerebral malaria
Abstract
Murine cerebral malaria (CM) induced by Plasmodium berghei ANKA kills susceptible mice within 24 to 48 h of onset of symptoms and is characterized by the production of inflammatory cytokines in the brain. C57BL/6J mice are sensitive to lethal CM, while A/J mice are resistant. These strains of mice were immunized with an adjuvant vaccine of killed whole-blood-stage parasites. The immunization protected C57BL/6 mice from lethal CM following virulent challenge. The same immunization increased the incidence of lethal CM in A/J mice challenged similarly. Histopathologic examination of the brains of mice from these studies revealed two distinct types of lesions. Type I CM is acute in onset; usually lethal; and characterized by widespread microglial activation, endothelial cell damage, and microvascular disruption in the brain. Type II CM is characterized by intense, but focal, mononuclear cell inflammation without endothelial cell damage or microvascular destruction. Animals with type II lesions were clinically normal and protected from type I lesions. Available clinical, epidemiological, and biochemical evidence suggests that type I and type II lesions might exist in human CM as well.
Figures
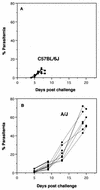

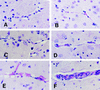
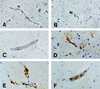
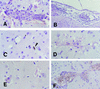
References
-
- Adams C W, Poston R N. Macrophage histology in paraffin-embedded multiple sclerosis plaques is demonstrated by the monoclonal panmacrophage marker HAM-56: correlation with chronicity of the lesion. Acta Neuropathol. 1990;80:208–211. - PubMed
-
- Allen S, Genton B, Alexander N, Gibson N, Raiko A. Malaria vaccine in children under 12 months of age. Lancet. 1995;346:1555–1556. - PubMed
-
- Clark I A, Ilschner S, MacMicking J D, Cowden W B. TNF and Plasmodium berghei ANKA-induced cerebral malaria. Immunol Lett. 1990;25:195–198. - PubMed
-
- Curfs J H, Hermsen C C, Meuwissen J H, Eling W M. Immunization against cerebral pathology in Plasmodium berghei-infected mice. Parasitology. 1992;105:7–14. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

