Transgenic cattle produced by reverse-transcribed gene transfer in oocytes
- PMID: 9826647
- PMCID: PMC24320
- DOI: 10.1073/pnas.95.24.14028
Transgenic cattle produced by reverse-transcribed gene transfer in oocytes
Abstract
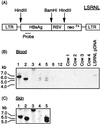
A critical requirement for integration of retroviruses, other than HIV and possibly related lentiviruses, is the breakdown of the nuclear envelope during mitosis. Nuclear envelope breakdown occurs during mitotic M-phase, the envelope reforming immediately after cell division, thereby permitting the translocation of the retroviral preintegration complex into the nucleus and enabling integration to proceed. In the oocyte, during metaphase II (MII) of the second meiosis, the nuclear envelope is also absent and the oocyte remains in MII arrest for a much longer period of time compared with M-phase in a somatic cell. Pseudotyped replication-defective retroviral vector was injected into the perivitelline space of bovine oocytes during MII. We show that reverse-transcribed gene transfer can take place in an oocyte in MII arrest of meiosis, leading to production of offspring, the majority of which are transgenic. We discuss the implications of this mechanism both as a means of production of transgenic livestock and as a model for naturally occurring recursive transgenesis.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

