Interferon-beta gene therapy inhibits tumor formation and causes regression of established tumors in immune-deficient mice
- PMID: 9826714
- PMCID: PMC24387
- DOI: 10.1073/pnas.95.24.14411
Interferon-beta gene therapy inhibits tumor formation and causes regression of established tumors in immune-deficient mice
Abstract
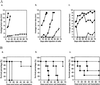
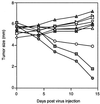
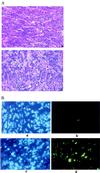
Despite the potential of type 1 interferons (IFNs) for the treatment of cancer, clinical experience with IFN protein therapy of solid tumors has been disappointing. IFN-beta has potent antiproliferative activity against most human tumor cells in vitro in addition to its known immunomodulatory activities. The antiproliferative effect, however, relies on IFN-beta concentrations that cannot be achieved by parenteral protein administration because of rapid protein clearance and systemic toxicities. We demonstrate here that ex vivo IFN-beta gene transduction by a replication-defective adenovirus in as few as 1% of implanted cells blocked tumor formation. Direct in vivo IFN-beta gene delivery into established tumors generated high local concentrations of IFN-beta, inhibited tumor growth, and in many cases caused complete tumor regression. Because the mice were immune-deficient, it is likely that the anti-tumor effect was primarily through direct inhibition of tumor cell proliferation and survival. Based on these studies, we argue that local IFN-beta gene therapy with replication-defective adenoviral vectors might be an effective treatment for some solid tumors.
Figures

Similar articles
-
Mesenchymal stem cells: potential precursors for tumor stroma and targeted-delivery vehicles for anticancer agents.J Natl Cancer Inst. 2004 Nov 3;96(21):1593-603. doi: 10.1093/jnci/djh299. J Natl Cancer Inst. 2004. PMID: 15523088
-
Treatment of a human breast cancer xenograft with an adenovirus vector containing an interferon gene results in rapid regression due to viral oncolysis and gene therapy.Proc Natl Acad Sci U S A. 1996 Apr 30;93(9):4513-8. doi: 10.1073/pnas.93.9.4513. Proc Natl Acad Sci U S A. 1996. PMID: 8633100 Free PMC article.
-
Human and mouse IFN-beta gene therapy exhibits different anti-tumor mechanisms in mouse models.Mol Ther. 2001 Oct;4(4):356-64. doi: 10.1006/mthe.2001.0464. Mol Ther. 2001. PMID: 11592839
-
Systemic IFN-beta gene therapy results in long-term survival in mice with established colorectal liver metastases.J Clin Invest. 2001 Jul;108(1):83-95. doi: 10.1172/JCI9841. J Clin Invest. 2001. PMID: 11435460 Free PMC article.
-
Inhibition of tumorigenicity and metastasis of human bladder cancer growing in athymic mice by interferon-beta gene therapy results partially from various antiangiogenic effects including endothelial cell apoptosis.Clin Cancer Res. 2002 Apr;8(4):1258-70. Clin Cancer Res. 2002. PMID: 11948141
Cited by
-
Anti-colorectal cancer effect of interleukin-2 and interferon-β fusion gene driven by carcinoembryonic antigen promoter.Onco Targets Ther. 2016 May 30;9:3259-67. doi: 10.2147/OTT.S97444. eCollection 2016. Onco Targets Ther. 2016. PMID: 27313471 Free PMC article.
-
Glioblastoma Treatment Modalities besides Surgery.J Cancer. 2019 Aug 27;10(20):4793-4806. doi: 10.7150/jca.32475. eCollection 2019. J Cancer. 2019. PMID: 31598150 Free PMC article. Review.
-
Novel Pegylated Interferon for the Treatment of Chronic Viral Hepatitis.Viruses. 2022 May 25;14(6):1128. doi: 10.3390/v14061128. Viruses. 2022. PMID: 35746606 Free PMC article. Review.
-
Conventional DNA-Damaging Cancer Therapies and Emerging cGAS-STING Activation: A Review and Perspectives Regarding Immunotherapeutic Potential.Cancers (Basel). 2023 Aug 16;15(16):4127. doi: 10.3390/cancers15164127. Cancers (Basel). 2023. PMID: 37627155 Free PMC article. Review.
-
Allogeneic stem cells engineered to release interferon β and scFv-PD1 target glioblastoma and alter the tumor microenvironment.Cytotherapy. 2024 Oct;26(10):1217-1226. doi: 10.1016/j.jcyt.2024.05.012. Epub 2024 May 17. Cytotherapy. 2024. PMID: 38852095
References
-
- Lengyel P. Annu Rev Biochem. 1982;51:251–282. - PubMed
-
- Belardelli F, Gresser I. Immunol Today. 1996;17:369–372. - PubMed
-
- Einhorn S, Grandér D. J Interferon Cytokine Res. 1996;16:275–281. - PubMed
-
- Wadler S, Schwartz E L. Cancer Res. 1990;50:3473–3486. - PubMed
-
- Tough D F, Borrow P, Sprent J. Science. 1996;272:1947–1950. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

