Serotonin receptor 1A knockout: an animal model of anxiety-related disorder
- PMID: 9826725
- PMCID: PMC24398
- DOI: 10.1073/pnas.95.24.14476
Serotonin receptor 1A knockout: an animal model of anxiety-related disorder
Abstract
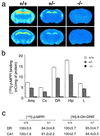
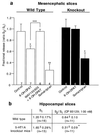
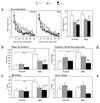
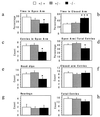
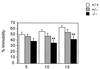
To investigate the contribution of individual serotonin (5-hydroxytryptamine; 5-HT) receptors to mood control, we have used homologous recombination to generate mice lacking specific serotonergic receptor subtypes. In the present report, we demonstrate that mice without 5-HT1A receptors display decreased exploratory activity and increased fear of aversive environments (open or elevated spaces). 5-HT1A knockout mice also exhibited a decreased immobility in the forced swim test, an effect commonly associated with antidepressant treatment. Although 5-HT1A receptors are involved in controlling the activity of serotonergic neurons, 5-HT1A knockout mice had normal levels of 5-HT and 5-hydroxyindoleacetic acid, possibly because of an up-regulation of 5-HT1B autoreceptors. Heterozygote 5-HT1A mutants expressed approximately one-half of wild-type receptor density and displayed intermediate phenotypes in most behavioral tests. These results demonstrate that 5-HT1A receptors are involved in the modulation of exploratory and fear-related behaviors and suggest that reductions in 5-HT1A receptor density due to genetic defects or environmental stressors might result in heightened anxiety.
Figures
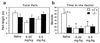
Comment in
-
Serotonin receptor knockouts: a moody subject.Proc Natl Acad Sci U S A. 1998 Dec 22;95(26):15153-4. doi: 10.1073/pnas.95.26.15153. Proc Natl Acad Sci U S A. 1998. PMID: 9860934 Free PMC article. Review. No abstract available.
References
-
- Hamon M. In: Serotoninergic Neurons and 5-HT Receptors in the CNS. Baumgarten H G, Göthert M, editors. Berlin: Springer; 1997. pp. 238–268.
-
- Tunnicliff G. Pharmacol Toxicol (Copenhagen) 1991;69:149–156. - PubMed
-
- Artigas F, Perez V, Alvarez E. Arch Gen Psychiatry. 1994;51:248–251. - PubMed
-
- Whitaker-Azmitia P M. Pharmacol Rev. 1991;43:553–561. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

