Antibodies to von Willebrand factor-cleaving protease in acute thrombotic thrombocytopenic purpura
- PMID: 9828246
- PMCID: PMC3159001
- DOI: 10.1056/NEJM199811263392203
Antibodies to von Willebrand factor-cleaving protease in acute thrombotic thrombocytopenic purpura
Abstract
Background: Thrombotic thrombocytopenic purpura is a potentially fatal disease characterized by widespread platelet thrombi in the microcirculation. In the normal circulation, von Willebrand factor is cleaved by a plasma protease. We explored the hypothesis that a deficiency of this protease predisposes patients with thrombotic thrombocytopenic purpura to platelet thrombosis.
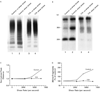
Methods: We studied the activity of von Willebrand factor-cleaving protease and sought inhibitors of this protease in plasma from patients with acute thrombotic thrombocytopenic purpura, patients with other diseases, and normal control subjects. We also investigated the effect of shear stress on the ristocetin cofactor activity of purified von Willebrand factor in the cryosupernatant fraction of the plasma samples.
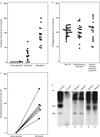
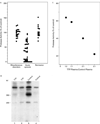
Results: Thirty-nine samples of plasma from 37 patients with acute thrombotic thrombocytopenic purpura had severe deficiency of von Willebrand factor-cleaving protease. No deficiency was detected in 16 samples of plasma from patients with thrombotic thrombocytopenic purpura in remission or in 74 plasma samples from normal subjects, randomly selected hospitalized patients or outpatients, or patients with hemolysis, thrombocytopenia, or thrombosis from other causes. Inhibitory activity against the protease was detected in 26 of the 39 plasma samples (67 percent) obtained during the acute phase of the disease. The inhibitors were IgG antibodies. Shear stress increased the ristocetin cofactor activity of von Willebrand factor in the cryosupernatant of plasma samples obtained during the acute phase, but decreased the activity in cryosupernatant of plasma from normal subjects.
Conclusions: Inhibitory antibodies against von Willebrand factor-cleaving protease occur in patients with acute thrombotic thrombocytopenic purpura. A deficiency of this protease is likely to have a critical role in the pathogenesis of platelet thrombosis in this disease.
Figures



Comment in
-
Moschcowitz, multimers, and metalloprotease.N Engl J Med. 1998 Nov 26;339(22):1629-31. doi: 10.1056/NEJM199811263392210. N Engl J Med. 1998. PMID: 9828253 No abstract available.
-
von Willebrand factor-cleaving protease in thrombotic thrombocytopenic purpura and the hemolytic-uremic syndrome.N Engl J Med. 1999 Apr 29;340(17):1368-9. N Engl J Med. 1999. PMID: 10223877 No abstract available.
References
-
- Moschcowitz E. Hyaline thrombosis of the terminal arterioles and capillaries: a hitherto undescribed disease. Proc N Y Pathol Soc. 1924;24:21–24.
-
- Baehr G, Klemperer P, Schifrin A. Acute febrile anemia and thrombocytopenic purpura with diffuse platelet thromboses of capillaries and arterioles. Trans Am Physicians. 1936;51:43–58.
-
- Rock GA, Shumak KH, Buskard NA, et al. Comparison of plasma exchange with plasma infusion in the treatment of thrombotic thrombocytopenic purpura. N Engl J Med. 1991;325:393–397. - PubMed
-
- Török TJ, Holman RC, Chorba TL. Increasing mortality from thrombotic thrombocytopenic purpura in the United States — analysis of national mortality data, 1968–1991. Am J Hematol. 1995;50:84–90. - PubMed
-
- Bell WR. Thrombotic thrombocytopenic purpura/hemolytic uremic syndrome relapse: frequency, pathogenesis, and meaning. Semin Hematol. 1997;34:134–139. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
