Expression patterns of cyclin D1 and related proteins regulating G1-S phase transition in uveal melanoma and retinoblastoma
- PMID: 9828785
- PMCID: PMC1722705
- DOI: 10.1136/bjo.82.8.961
Expression patterns of cyclin D1 and related proteins regulating G1-S phase transition in uveal melanoma and retinoblastoma
Abstract
Background/aims: A checkpoint mechanism in late G1, whose regulation via loss of retinoblastoma protein (pRB) or p16, or overexpression of cyclin D1 or cyclin dependent kinase 4 (CDK4), has been proposed to constitute a common pathway to malignancy. The aims of this study were (a) to compare markers of cell cycle G1-S phase transition in an intraocular tumour with known pRB deficiency (retinoblastoma) and compare it with one with an apparently functional pRB (uveal melanoma); (b) to determine if one of these markers may have a role in the pathogenesis of uveal melanoma; and (c) to determine if there is a difference in cell cycle marker expression following treatment of uveal melanoma and retinoblastoma.
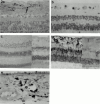
Methods: 90 eyes were enucleated from 89 patients for retinoblastoma (n = 24) or for choroidal or ciliary body melanoma (n = 66). Conventional paraffin sections were assessed for cell type and degree of differentiation. Additional slides were investigated applying standard immunohistochemical methods with antibodies specific for cyclin D1 protein, pRB, p53, p21, p16, BCL-2, and MIB-1.
Results: Cyclin D1 protein and pRB were negative in retinoblastoma using the applied antibodies. In contrast, cyclin D1 protein expression was observed in 65% of uveal melanomas; a positive correlation between cyclin D1 cell positivity and tumour cell type, location, growth fraction, as well as with pRB positivity was observed. p53, p21, and p16 could be demonstrated in both tumours. An inverse relation between p53 and p21 expression was demonstrated in most choroidal melanomas and in some retinoblastomas. Apart from a decrease in the growth fractions of the tumours as determined by MIB-1, a significant difference in the expression of G1-S phase transition markers in vital areas of uveal melanoma and retinoblastoma following treatment with radiotherapy and/or chemotherapy was not observed.
Conclusion: Retinoblastomas and uveal melanomas, two tumours of differing pRB status, differ also in their immunohistochemical pattern for markers of the G1-S phase transition of the cell cycle. The results of the present study support the concept of (a) an autoregulatory loop between pRB and cyclin D1 in tumours with a functional pRB and the disruption of this loop in the presence of pRB mutation, as well as (b) a checkpoint mechanism in late G1, whose regulation via loss of p16 or pRB, or overexpression of cyclin D1 constitutes a common pathway to malignancy. Further, the results raise the possibility of cyclin D1 overexpression having a role in the pathogenesis of uveal melanoma.
Figures


Similar articles
-
Retinoblastoma protein in human renal cell carcinoma in relation to alterations in G1/S regulatory proteins.Int J Cancer. 2004 Mar 20;109(2):189-93. doi: 10.1002/ijc.11665. Int J Cancer. 2004. PMID: 14750168
-
Aberrant expression of G(1)/S regulators is a frequent event in sporadic pituitary adenomas.Carcinogenesis. 2001 Aug;22(8):1149-54. doi: 10.1093/carcin/22.8.1149. Carcinogenesis. 2001. PMID: 11470742
-
Altered expression of Rb and p53 in uveal melanomas following plaque radiotherapy.Am J Ophthalmol. 2002 Feb;133(2):242-8. doi: 10.1016/s0002-9394(01)01362-9. Am J Ophthalmol. 2002. PMID: 11812429
-
Eye cancer: unique insights into oncogenesis: the Cogan Lecture.Invest Ophthalmol Vis Sci. 2006 May;47(5):1736-45. doi: 10.1167/iovs.05-1291. Invest Ophthalmol Vis Sci. 2006. PMID: 16638975 Free PMC article. Review. No abstract available.
-
Cyclin D1 and human neoplasia.Mol Pathol. 1998 Feb;51(1):1-7. doi: 10.1136/mp.51.1.1. Mol Pathol. 1998. PMID: 9624412 Free PMC article. Review.
Cited by
-
p16(INK4a) expression in retinoblastoma: a marker of differentiation grade.Diagn Pathol. 2014 Dec 11;9:180. doi: 10.1186/s13000-014-0180-1. Diagn Pathol. 2014. PMID: 25499675 Free PMC article.
-
Dual Targeting of CDK4/6 and cMET in Metastatic Uveal Melanoma.Cancers (Basel). 2021 Mar 4;13(5):1104. doi: 10.3390/cancers13051104. Cancers (Basel). 2021. PMID: 33806615 Free PMC article.
-
Telomerase expression in uveal melanoma.Br J Ophthalmol. 2000 Feb;84(2):217-23. doi: 10.1136/bjo.84.2.217. Br J Ophthalmol. 2000. PMID: 10655201 Free PMC article.
-
Cyclin-Dependent Kinase Inhibitors in the Rare Subtypes of Melanoma Therapy.Molecules. 2024 Nov 6;29(22):5239. doi: 10.3390/molecules29225239. Molecules. 2024. PMID: 39598629 Free PMC article. Review.
-
Dual inhibition of protein kinase C and p53-MDM2 or PKC and mTORC1 are novel efficient therapeutic approaches for uveal melanoma.Oncotarget. 2016 Jun 7;7(23):33542-56. doi: 10.18632/oncotarget.9552. Oncotarget. 2016. PMID: 27507190 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous
