Neuropeptide-mediated excitability: a key triggering mechanism for seizure generation in the developing brain
- PMID: 9829688
- PMCID: PMC3372323
- DOI: 10.1016/s0166-2236(98)01275-2
Neuropeptide-mediated excitability: a key triggering mechanism for seizure generation in the developing brain
Abstract
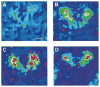
Most human seizures occur early in life,consistent with established excitability-promoting features of the developing brain. Surprisingly, the majority of developmental seizures are not spontaneous but are provoked by injurious or stressful stimuli. What mechanisms mediate'triggering' of seizures and limit such reactive seizures to early postnatal life? Recent evidence implicates the excitatory neuropeptide, corticotropin-releasing hormone (CRH). Stress activates expression of the CRH gene in several limbic regions, and CRH-expressing neurons are strategically localized in the immature rat hippocampus, in which this neuropeptide increases the excitability of pyramidal cells in vitro. Indeed, in vivo, activation of CRH receptors--maximally expressed in hippocampus and amygdala during the developmental period which is characterized by peak susceptibility to 'provoked' convulsions--induces severe, age-dependent seizures. Thus, converging data indicate that activation of expression of CRH constitutes an important mechanism for generating developmentally regulated, triggered seizures, with considerable clinical relevance.
Figures
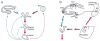


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

