Characterization of Proteus mirabilis precocious swarming mutants: identification of rsbA, encoding a regulator of swarming behavior
- PMID: 9829920
- PMCID: PMC107696
- DOI: 10.1128/JB.180.23.6126-6139.1998
Characterization of Proteus mirabilis precocious swarming mutants: identification of rsbA, encoding a regulator of swarming behavior
Abstract
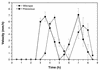
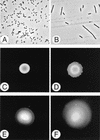
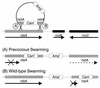
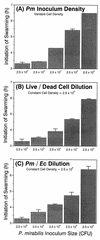
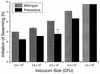
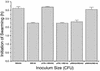
Proteus mirabilis swarming behavior is characterized by the development of concentric rings of growth that are formed as cyclic events of swarmer cell differentiation, swarming migration, and cellular differentiation are repeated during colony translocation across a surface. This cycle produces the bull's-eye colony often associated with cultures of P. mirabilis. How the cells communicate with one another to coordinate these perfectly synchronized rings is presently unknown. We report here the identification of a genetic locus that, when mutated, results in a precocious swarming phenotype. These mutants are defective in the temporal control of swarming migration and start swarming ca. 60 min sooner than wild-type cells. Unlike the wild type, precocious swarming mutants are also constitutive swarmer cells and swarm on minimal agar medium. The defects were found to be localized to a 5.4-kb locus on the P. mirabilis genome encoding RsbA (regulator of swarming behavior) and the P. mirabilis homologs to RcsB and RcsC. RsbA is homologous to membrane sensor histidine kinases of the two-component family of regulatory proteins, suggesting that RsbA may function as a sensor of environmental conditions required to initiate swarming migration. Introduction of a rsbA mutation back into the wild type via allelic-exchange mutagenesis reconstructed the precocious swarming phenotype, which could be complemented in trans by a plasmid-borne copy of rsbA. Overexpression of RsbA in wild-type cells resulted in precocious swarming, suggesting that RsbA may have both positive and negative functions in regulating swarming migration. A possible model to describe the role of RsbA in swarming migration is discussed.
Figures




References
-
- Alex L A, Simon M I. Protein histidine kinases and signal transduction in prokaryotes and eukaryotes. Trends Genet. 1994;10:133–138. - PubMed
-
- Allison C, Lai H C, Gygi D, Hughes C. Cell differentiation of Proteus mirabilis is initiated by glutamine, a specific chemoattractant for swarming cells. Mol Microbiol. 1993;8:53–60. - PubMed
-
- Altschul S, Gish W, Miller W, Myers E, Lipman D. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. - PubMed
-
- Arricau N, Hermant D, Waxin H, Ecobichon C, Duffey P, Popoff M. The RcsB-RcsC regulatory system of Salmonella typhi differentially modulates the expression of invasion proteins, flagellin and Vi antigen in response to osmolarity. Mol Microbiol. 1998;29:835–850. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources

