Shewanella putrefaciens mtrB encodes an outer membrane protein required for Fe(III) and Mn(IV) reduction
- PMID: 9829939
- PMCID: PMC107715
- DOI: 10.1128/JB.180.23.6292-6297.1998
Shewanella putrefaciens mtrB encodes an outer membrane protein required for Fe(III) and Mn(IV) reduction
Abstract
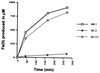
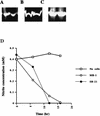
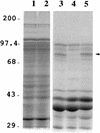
Iron and manganese oxides or oxyhydroxides are abundant transition metals, and in aquatic environments they serve as terminal electron acceptors for a large number of bacterial species. The molecular mechanisms of anaerobic metal reduction, however, are not understood. Shewanella putrefaciens is a facultative anaerobe that uses Fe(III) and Mn(IV) as terminal electron acceptors during anaerobic respiration. Transposon mutagenesis was used to generate mutants of S. putrefaciens, and one such mutant, SR-21, was analyzed in detail. Growth and enzyme assays indicated that the mutation in SR-21 resulted in loss of Fe(III) and Mn(IV) reduction but did not affect its ability to reduce other electron acceptors used by the wild type. This deficiency was due to Tn5 inactivation of an open reading frame (ORF) designated mtrB. mtrB encodes a protein of 679 amino acids and contains a signal sequence characteristic of secreted proteins. Analysis of membrane fractions of the mutant, SR-21, and wild-type cells indicated that MtrB is located on the outer membrane of S. putrefaciens. A 5.2-kb DNA fragment that contains mtrB was isolated and completely sequenced. A second ORF, designated mtrA, was found directly upstream of mtrB. The two ORFs appear to be arranged in an operon. mtrA encodes a putative 10-heme c-type cytochrome of 333 amino acids. The N-terminal sequence of MtrA contains a potential signal sequence for secretion across the cell membrane. The amino acid sequence of MtrA exhibited 34% identity to NrfB from Escherichia coli, which is involved in formate-dependent nitrite reduction. To our knowledge, this is the first report of genes encoding proteins involved in metal reduction.
Figures


References
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

