Synaptonemal complex morphogenesis and sister-chromatid cohesion require Mek1-dependent phosphorylation of a meiotic chromosomal protein
- PMID: 9832507
- PMCID: PMC317243
- DOI: 10.1101/gad.12.22.3551
Synaptonemal complex morphogenesis and sister-chromatid cohesion require Mek1-dependent phosphorylation of a meiotic chromosomal protein
Abstract
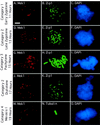
Development of yeast meiotic chromosome cores into full-length synaptonemal complexes requires the MEK1 gene product, a meiosis-specific protein kinase homolog. The Mek1 protein associates with meiotic chromosomes and colocalizes with the Red1 protein, which is a component of meiotic chromosome cores. Mek1 and Red1 interact physically in meiotic cells, as demonstrated by coimmunoprecipitation and the two-hybrid protein system. Hop1, another protein associated with meiotic chromosome cores, also interacts with Mek1 but only in the presence of Red1. Red1 displays Mek1-dependent phosphorylation, both in vitro and in vivo, and Mek1 kinase activity is necessary for Mek1 function in vivo. Fluorescent in situ hybridization analysis indicates that Mek1-mediated phosphorylation of Red1 is required for meiotic sister-chromatid cohesion, raising the possibility that cohesion is regulated by protein phosphorylation.
Figures



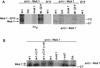

References
-
- Bai C, Elledge SJ. Gene identification using the yeast two-hybrid system. Methods Enzymol. 1997;283:141–156. - PubMed
-
- Bishop D, Park D, Xu L, Kleckner N. DMC1: A meiosis-specific yeast homolog of E. coli recA required for recombination, synaptonemal complex formation, and cell cycle progression. Cell. 1992;69:439–456. - PubMed
-
- Brunelli JP, Pall ML. A series of yeast shuttle vectors for the expression of cDNAs and other DNA sequences. Yeast. 1993;9:1299–1308. - PubMed
-
- Burns N, Grimwade B, Ross-Macdonald PB, Choi E-Y, Finberg K, Roeder GS, Snyder M. Large-scale analysis of gene expression, protein localization and gene disruption in Saccharomyces cerevisiae. Genes & Dev. 1994;8:1087–1105. - PubMed
-
- Chua PR, Roeder GS. Zip2, a meiosis-specific protein required for the initiation of chromosome synapsis. Cell. 1998;93:349–359. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
