Unc-45 mutations in Caenorhabditis elegans implicate a CRO1/She4p-like domain in myosin assembly
- PMID: 9832550
- PMCID: PMC2133068
- DOI: 10.1083/jcb.143.5.1215
Unc-45 mutations in Caenorhabditis elegans implicate a CRO1/She4p-like domain in myosin assembly
Abstract
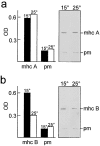
The Caenorhabditis elegans unc-45 locus has been proposed to encode a protein machine for myosin assembly. The UNC-45 protein is predicted to contain an NH2-terminal domain with three tetratricopeptide repeat motifs, a unique central region, and a COOH-terminal domain homologous to CRO1 and She4p. CRO1 and She4p are fungal proteins required for the segregation of other molecules in budding, endocytosis, and septation. Three mutations that lead to temperature-sensitive (ts) alleles have been localized to conserved residues within the CRO1/She4p-like domain, and two lethal alleles were found to result from stop codon mutations in the central region that would prevent translation of the COOH-terminal domain. Electron microscopy shows that thick filament accumulation in vivo is decreased by approximately 50% in the CB286 ts mutant grown at the restrictive temperature. The thick filaments that assemble have abnormal structure. Immunofluorescence and immunoelectron microscopy show that myosins A and B are scrambled, in contrast to their assembly into distinct regions at the permissive temperature and in wild type. This abnormal structure correlates with the high degree of instability of the filaments in vitro as reflected by their extremely low yields and shortened lengths upon isolation. These results implicate the UNC-45 CRO1/She4p-like region in the assembly of myosin isoforms in C. elegans and suggest a possible common mechanism for the function of this UCS (UNC-45/CRO1/She4p) protein family.
Figures







References
-
- Altschul SF, Gish W, Miller W, Myers EW, Lipman DJ. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. - PubMed
-
- Bernstein SI, Mogami K, Donady JJ, Emerson CP., Jr Drosophilamuscle myosin heavy chain encoded by a single gene in a cluster of muscle mutations. Nature. 1983;302:393–397. - PubMed
-
- Berteaux-Lecellier V, Zickler D, Debuchy R, Panvier-Adoutte A, Thompson-Coffe C, Picard M. A homologue of the yeast SHE4 gene is essential for the transition between the syncytial and cellular stages during sexual reproduction of the fungus Podospora anserina. . EMBO (Eur Mol Biol Organ) J. 1998;17:1248–1258. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

