The "8-kD" cytoplasmic dynein light chain is required for nuclear migration and for dynein heavy chain localization in Aspergillus nidulans
- PMID: 9832552
- PMCID: PMC2133080
- DOI: 10.1083/jcb.143.5.1239
The "8-kD" cytoplasmic dynein light chain is required for nuclear migration and for dynein heavy chain localization in Aspergillus nidulans
Abstract
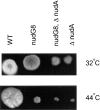
The heavy chain of cytoplasmic dynein is required for nuclear migration in Aspergillus nidulans and other fungi. Here we report on a new gene required for nuclear migration, nudG, which encodes a homologue of the "8-kD" cytoplasmic dynein light chain (CDLC). We demonstrate that the temperature sensitive nudG8 mutation inhibits nuclear migration and growth at restrictive temperature. This mutation also inhibits asexual and sexual sporulation, decreases the intracellular concentration of the nudG CDLC protein and causes the cytoplasmic dynein heavy chain to be absent from the mycelial tip, where it is normally located in wild-type mycelia. Coimmunoprecipitation experiments with antibodies against the cytoplasmic dynein heavy chain (CDHC) and the nudG CDLC demonstrated that some fraction of the cytoplasmic dynein light chain is in a protein complex with the CDHC. Sucrose gradient sedimentation analysis, however, showed that not all of the NUDG protein is complexed with the heavy chain. A double mutant carrying a cytoplasmic dynein heavy chain deletion plus a temperature-sensitive nudG mutation grew no more slowly at restrictive temperature than a strain with only the CDHC deletion. This result demonstrates that the effect of the nudG mutation on nuclear migration and growth is mediated through an interaction with the CDHC rather than with some other molecule (e.g., myosin-V) with which the 8-kD CDLC might theoretically interact.
Figures




References
-
- Allan V. Organelle movement. Dynactin: portrait of dynein regulator. Curr Biol. 1994;4:1000–1002. - PubMed
-
- Andersland JM, Fisher DD, Wymer CL, Cyr RJ, Parthasarathy MV. Characterization of a monoclonal antibody prepared against plant actin. Cell Motil Cytoskel. 1994;29:339–344. - PubMed
-
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding . Anal Biochem. 1976;72:248–254. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

