The kakapo mutation affects terminal arborization and central dendritic sprouting of Drosophila motorneurons
- PMID: 9832556
- PMCID: PMC2133088
- DOI: 10.1083/jcb.143.5.1283
The kakapo mutation affects terminal arborization and central dendritic sprouting of Drosophila motorneurons
Abstract
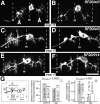
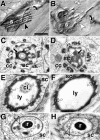
The lethal mutation l(2)CA4 causes specific defects in local growth of neuronal processes. We uncovered four alleles of l(2)CA4 and mapped it to bands 50A-C on the polytene chromosomes and found it to be allelic to kakapo (. Genetics. 146:275- 285). In embryos carrying our kakapo mutant alleles, motorneurons form correct nerve branches, showing that long distance growth of neuronal processes is unaffected. However, neuromuscular junctions (NMJs) fail to form normal local arbors on their target muscles and are significantly reduced in size. In agreement with this finding, antibodies against kakapo (Gregory and Brown. 1998. J. Cell Biol. 143:1271-1282) detect a specific epitope at all or most Drosophila NMJs. Within the central nervous system of kakapo mutant embryos, neuronal dendrites of the RP3 motorneuron form at correct positions, but are significantly reduced in size. At the subcellular level we demonstrate two phenotypes potentially responsible for the defects in neuronal branching: first, transmembrane proteins, which can play important roles in neuronal growth regulation, are incorrectly localized along neuronal processes. Second, microtubules play an important role in neuronal growth, and kakapo appears to be required for their organization in certain ectodermal cells: On the one hand, kakapo mutant embryos exhibit impaired microtubule organization within epidermal cells leading to detachment of muscles from the cuticle. On the other, a specific type of sensory neuron (scolopidial neurons) shows defects in microtubule organization and detaches from its support cells.
Figures





References
-
- Angau-Petit D, Toth P, Rogero O, Faille L, Tejedor FJ, Ferrús A. Enhanced neurotransmitter release is associated with reduction of neuronal branching in a Drosophilamutant overexpressing frequenin. Eur J Neurosci. 1998;10:423–434. - PubMed
-
- Arcaro KF, Lnenicka GA. Intrinsic differences in axonal growth from crayfish fast and slow motorneurons. Dev Biol. 1995;168:272–283. - PubMed
-
- Ashburner M, Faithfull J, Littlewood T, Richards G, Smith S, Velissariou V, Woodruff RC. Report of new mutants—Drosophila melanogaster. . Dros Info Service. 1980;55:193–195.
-
- Avila J, Domínguez J, Díaz-Nido J. Regulation of microtubule dynamics by microtubule-associated protein expression and phosphorylation during neuronal development. Int J Dev Biol. 1994;38:13–25. - PubMed
-
- Bate, M. 1993. The mesoderm and its derivatives. In The Development of Drosophila melanogaster. M. Bate and A. Martínez Arias, editors. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY. 1013–1090.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

