AnkyrinG is required for clustering of voltage-gated Na channels at axon initial segments and for normal action potential firing
- PMID: 9832557
- PMCID: PMC2133082
- DOI: 10.1083/jcb.143.5.1295
AnkyrinG is required for clustering of voltage-gated Na channels at axon initial segments and for normal action potential firing
Abstract
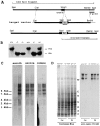
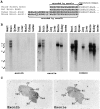
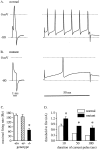
Voltage-gated sodium channels (NaCh) are colocalized with isoforms of the membrane-skeletal protein ankyrinG at axon initial segments, nodes of Ranvier, and postsynaptic folds of the mammalian neuromuscular junction. The role of ankyrinG in directing NaCh localization to axon initial segments was evaluated by region-specific knockout of ankyrinG in the mouse cerebellum. Mutant mice exhibited a progressive ataxia beginning around postnatal day P16 and subsequent loss of Purkinje neurons. In mutant mouse cerebella, NaCh were absent from axon initial segments of granule cell neurons, and Purkinje cells showed deficiencies in their ability to initiate action potentials and support rapid, repetitive firing. Neurofascin, a member of the L1CAM family of ankyrin-binding cell adhesion molecules, also exhibited impaired localization to initial segments of Purkinje cell neurons. These results demonstrate that ankyrinG is essential for clustering NaCh and neurofascin at axon initial segments and is required for physiological levels of sodium channel activity.
Figures





References
-
- Armstrong CM, Hille B .Voltage-gated ion channels and electrical excitability. Neuron. 1998;20:371–380. - PubMed
-
- Bennett V, Lambert S, Davis JQ, Zhang X. Molecular architecture of the specialized axonal membrane at the node of Ranvier. Soc Gen Physiol Ser. 1997;52:107–120. - PubMed
-
- Boakes RJ, Candy JM, Dick DJ, Harris JB, Pollard S, Thompson J. Abnormal electrophysiological and anatomical characteristics of cerebellar Purkinje cells in the mutant mouse jolting. J Physiol (Lond) 1984;346:27P. - PubMed
-
- Burgess DL, Kohrman DC, Galt J, Plummer NW, Jones JM, Spear B, Meisler MH. Mutation of a new sodium channel gene, Scn8a, in the mouse mutant “motor endplate disease.” . Nat Genet. 1995;10:461–465. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

