Direct assessment of peripheral pharmacokinetics in humans: comparison between cantharides blister fluid sampling, in vivo microdialysis and saliva sampling
- PMID: 9833594
- PMCID: PMC1873691
- DOI: 10.1046/j.1365-2125.1998.00805.x
Direct assessment of peripheral pharmacokinetics in humans: comparison between cantharides blister fluid sampling, in vivo microdialysis and saliva sampling
Abstract
Aims: Skin blister fluid sampling, in vivo microdialysis and saliva sampling are commonly employed as surrogates for the measurement of drug concentrations in peripheral compartments. Although expected to exhibit comparable results, data derived from these techniques have never been directly compared. Thus, the aim of the present study was to evaluate the comparability of these techniques.
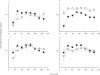
Methods: Paracetamol, a model drug with low protein binding, was administered to seven healthy volunteers at an oral dose of 2000 mg. Subsequently, tissue kinetics were measured simultaneously in cantharides induced skin blisters, microdialysates of subcutaneous- and skeletal muscle-tissue and saliva and compared to serum concentrations.
Results: Mean ratio (AUCblister/AUCserum) was 0.88 (95% CI, 0.50-1.26), mean ratio (AUCmuscle/AUCserum) was 1.08 (0.67-1.49), mean ratio (AUCsubcutaneous/AUCserum) was 0.96 (0.41-1.51) and mean ratio (AUCsaliva/AUCserum) was 1.83 (1.39-2.27). In this study the concentration profiles after single oral administration differed among the three methods. The time course of the concentration (peripheral compartment)/concentration (serum)-ratios showed that cantharides blister and microdialysate concentrations closely paralleled serum levels. An equilibration period of less than 2 h had to be taken into account for blister measurements. In contrast, saliva concentrations were significantly higher than corresponding serum concentrations.
Conclusions: Skin blister sampling and microdialysis closely mirrored corresponding serum concentrations and, thus, proved to be suitable techniques for the assessment of peripheral compartment pharmacokinetics. In contrast, saliva data overestimated the corresponding serum concentrations.
Figures


Similar articles
-
Comparison of three different experimental methods for the assessment of peripheral compartment pharmacokinetics in humans.Life Sci. 1998;62(15):PL227-34. doi: 10.1016/s0024-3205(98)00071-x. Life Sci. 1998. PMID: 9566779
-
Penetration of moxifloxacin into peripheral compartments in humans.Antimicrob Agents Chemother. 1999 Oct;43(10):2345-9. doi: 10.1128/AAC.43.10.2345. Antimicrob Agents Chemother. 1999. PMID: 10508004 Free PMC article. Clinical Trial.
-
Does cantharides blister fluid provide access to the peripheral compartment?Eur J Clin Pharmacol. 1982 Oct;23(4):327-30. doi: 10.1007/BF00613614. Eur J Clin Pharmacol. 1982. PMID: 7173302
-
Methods to measure target site penetration of antibiotics in critically ill patients.Curr Clin Pharmacol. 2013 Feb 1;8(1):46-58. Curr Clin Pharmacol. 2013. PMID: 22946872 Review.
-
Role of physical chemical properties in drug relay into skin compartments.Skin Pharmacol Physiol. 2008;21(6):294-9. doi: 10.1159/000148221. Epub 2008 Aug 1. Skin Pharmacol Physiol. 2008. PMID: 18679045 Review.
Cited by
-
Importance of relating efficacy measures to unbound drug concentrations for anti-infective agents.Clin Microbiol Rev. 2013 Apr;26(2):274-88. doi: 10.1128/CMR.00092-12. Clin Microbiol Rev. 2013. PMID: 23554417 Free PMC article. Review.
-
A multi-scale modeling framework for individualized, spatiotemporal prediction of drug effects and toxicological risk.Front Pharmacol. 2013 Jan 22;3:204. doi: 10.3389/fphar.2012.00204. eCollection 2012. Front Pharmacol. 2013. PMID: 23346056 Free PMC article.
-
Applicability of free drug hypothesis to drugs with good membrane permeability that are not efflux transporter substrates: A microdialysis study in rats.Pharmacol Res Perspect. 2020 Apr;8(2):e00575. doi: 10.1002/prp2.575. Pharmacol Res Perspect. 2020. PMID: 32266794 Free PMC article.
-
Issues in pharmacokinetics and pharmacodynamics of anti-infective agents: distribution in tissue.Antimicrob Agents Chemother. 2004 May;48(5):1441-53. doi: 10.1128/AAC.48.5.1441-1453.2004. Antimicrob Agents Chemother. 2004. PMID: 15105091 Free PMC article. Review. No abstract available.
-
Impaired neutrophil chemotaxis in Crohn's disease relates to reduced production of chemokines and can be augmented by granulocyte-colony stimulating factor.Aliment Pharmacol Ther. 2006 Aug 15;24(4):651-60. doi: 10.1111/j.1365-2036.2006.03016.x. Aliment Pharmacol Ther. 2006. PMID: 16907898 Free PMC article.
References
-
- Müller M, Mader RM, Steiner B, et al. 5-Fluorouracil kinetics in the interstitial tumor space: clinical response in breast cancer patients. Cancer Res. 1997;57:2598–2601. - PubMed
-
- Presant CA, Wolf W, Waluch V, et al. Association of intratumoral pharmacokinetics of fluorouracil with clinical response. Lancet. 1994;343:1184–1187. - PubMed
-
- Rolinson GN. Forty years of β-lactam research. J Antimicrob Chemother. 1998;43:589–603. - PubMed
-
- Wise R, Gillett AP, Cadge B, Durham SR, Baker S. The influence of protein binding upon tissue fluid levels of six ß-lactam antibiotics. J Infect Dis. 1980;142:77–82. - PubMed
-
- Müller M, Schmid R, Georgopoulos A, Buxbaum A, Wasicek C, Eichler HG. Application of microdialysis to clinical pharmacokinetics in humans. Clin Pharmacol Ther. 1995;57:371–380. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

