Single-channel properties of IKs potassium channels
- PMID: 9834139
- PMCID: PMC2229447
- DOI: 10.1085/jgp.112.6.665
Single-channel properties of IKs potassium channels
Erratum in
- J Gen Physiol 1999 Mar;113(3):505
Abstract
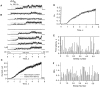
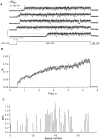
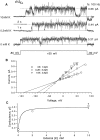
Expressed in Xenopus oocytes, KvLQT1 channel subunits yield a small, rapidly activating, voltage- dependent potassium conductance. When coexpressed with the minK gene product, a slowly activating and much larger potassium current results. Using fluctuation analysis and single-channel recordings, we have studied the currents formed by human KvLQT1 subunits alone and in conjunction with human or rat minK subunits. With low external K+, the single-channel conductances of these three channel types are estimated to be 0.7, 4.5, and 6.5 pS, respectively, based on noise analysis at 20 kHz bandwidth of currents at +50 mV. Power spectra computed over the range 0.1 Hz-20 kHz show a weak frequency dependence, consistent with current interruptions occurring on a broad range of time scales. The broad spectrum causes the apparent single-channel current value to depend on the bandwidth of the recording, and is mirrored in very "flickery" single-channel events of the channels from coexpressed KvLQT1 and human minK subunits. The increase in macroscopic current due to the presence of the minK subunit is accounted for by the increased apparent single-channel conductance it confers on the expressed channels. The rat minK subunit also confers the property that the outward single-channel current is increased by external potassium ions.
Figures







Similar articles
-
Single-channel characteristics of wild-type IKs channels and channels formed with two minK mutants that cause long QT syndrome.J Gen Physiol. 1998 Dec;112(6):651-63. doi: 10.1085/jgp.112.6.651. J Gen Physiol. 1998. PMID: 9834138 Free PMC article.
-
MinK subdomains that mediate modulation of and association with KvLQT1.J Gen Physiol. 2000 Sep;116(3):379-90. doi: 10.1085/jgp.116.3.379. J Gen Physiol. 2000. PMID: 10962015 Free PMC article.
-
MinK-KvLQT1 fusion proteins, evidence for multiple stoichiometries of the assembled IsK channel.J Biol Chem. 1998 Dec 18;273(51):34069-74. doi: 10.1074/jbc.273.51.34069. J Biol Chem. 1998. PMID: 9852064
-
The IKs channel: coassembly of IsK (minK) and KvLQT1 proteins.Rev Physiol Biochem Pharmacol. 1999;137:191-226. doi: 10.1007/3-540-65362-7_6. Rev Physiol Biochem Pharmacol. 1999. PMID: 10207306 Review. No abstract available.
-
Properties and regulation of the minK potassium channel protein.Physiol Rev. 1997 Jul;77(3):627-41. doi: 10.1152/physrev.1997.77.3.627. Physiol Rev. 1997. PMID: 9234960 Review.
Cited by
-
KCNQ1 channel modulation by KCNE proteins via the voltage-sensing domain.J Physiol. 2015 Jun 15;593(12):2617-25. doi: 10.1113/jphysiol.2014.287672. Epub 2015 Feb 16. J Physiol. 2015. PMID: 25603957 Free PMC article. Review.
-
KCNE1 enhances phosphatidylinositol 4,5-bisphosphate (PIP2) sensitivity of IKs to modulate channel activity.Proc Natl Acad Sci U S A. 2011 May 31;108(22):9095-100. doi: 10.1073/pnas.1100872108. Epub 2011 May 16. Proc Natl Acad Sci U S A. 2011. PMID: 21576493 Free PMC article.
-
LQT1 mutations in KCNQ1 C-terminus assembly domain suppress IKs using different mechanisms.Cardiovasc Res. 2014 Dec 1;104(3):501-11. doi: 10.1093/cvr/cvu231. Epub 2014 Oct 24. Cardiovasc Res. 2014. PMID: 25344363 Free PMC article.
-
Insights into Cardiac IKs (KCNQ1/KCNE1) Channels Regulation.Int J Mol Sci. 2020 Dec 11;21(24):9440. doi: 10.3390/ijms21249440. Int J Mol Sci. 2020. PMID: 33322401 Free PMC article. Review.
-
Single-channel basis for the slow activation of the repolarizing cardiac potassium current, I(Ks).Proc Natl Acad Sci U S A. 2013 Mar 12;110(11):E996-1005. doi: 10.1073/pnas.1214875110. Epub 2013 Feb 19. Proc Natl Acad Sci U S A. 2013. PMID: 23431135 Free PMC article.
References
-
- Aldrich RW, Corey DP, Stevens CF. A reinterpretation of mammalian sodium channel gating based on single channel recording. Nature. 1983;306:436–441. - PubMed
-
- Attali B, Guillemare E, Lesage F, Honore E, Romey G, Lazdunski M, Barhanin J. The protein IsK is a dual activator of K+ and Cl−channels. Nature. 1993;365:850–852. - PubMed
-
- Barhanin J, Lesage F, Guillemare E, Fink M, Lazdunski M, Romey G. K(V)LQT1 and lsK (minK) proteins associate to form the I(Ks) cardiac potassium current. Nature. 1996;384:78–80. - PubMed
-
- Ben-Efraim I, Shai Y, Attali B. Cytoplasmic and extracellular IsK peptides activate endogenous K+ and Cl− channels in Xenopusoocytes. Evidence for regulatory function. J Biol Chem. 1996;271:8768–8771. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

