Extracellular zinc ion inhibits ClC-0 chloride channels by facilitating slow gating
- PMID: 9834141
- PMCID: PMC2229451
- DOI: 10.1085/jgp.112.6.715
Extracellular zinc ion inhibits ClC-0 chloride channels by facilitating slow gating
Abstract
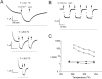
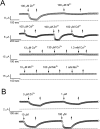
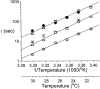
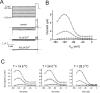
Extracellular Zn2+ was found to reversibly inhibit the ClC-0 Cl- channel. The apparent on and off rates of the inhibition were highly temperature sensitive, suggesting an effect of Zn2+ on the slow gating (or inactivation) of ClC-0. In the absence of Zn2+, the rate of the slow-gating relaxation increased with temperature, with a Q10 of approximately 37. Extracellular Zn2+ facilitated the slow-gating process at all temperatures, but the Q10 did not change. Further analysis of the rate constants of the slow-gating process indicates that the effect of Zn2+ is mostly on the forward rate (the rate of inactivation) rather than the backward rate (the rate of recovery from inactivation) of the slow gating. When ClC-0 is bound with Zn2+, the equilibrium constant of the slow-gating process is increased by approximately 30-fold, reflecting a 30-fold higher Zn2+ affinity in the inactivated channel than in the open-state channel. As examined through a wide range of membrane potentials, Zn2+ inhibits the opening of the slow gate with equal potency at all voltages, suggesting that a two-state model is inadequate to describe the slow-gating transition. Following a model originally proposed by Pusch and co-workers (Pusch, M., U. Ludewig, and T.J. Jentsch. 1997. J. Gen. Physiol. 109:105-116), the effect of Zn2+ on the activation curve of the slow gate can be well described by adding two constraints: (a) the dissociation constant for Zn2+ binding to the open channel is 30 microM, and (b) the difference in entropy between the open state and the transition state of the slow-gating process is increased by 27 J/ mol/ degreesK for the Zn2+-bound channel. These results together indicate that extracellular Zn2+ inhibits ClC-0 by facilitating the slow-gating process.
Figures





References
-
- Backx PH, Yue DT, Lawrence JH, Marban E, Tomaselli GF. Molecular localization of an ion-binding site within the pore of mammalian sodium channels. Science. 1992;257:248–251. - PubMed
-
- Baukrowitz T, Yellen G. Modulation of K+ current by frequency and external [K+]: a tale of two inactivation mechanisms. Neuron. 1995;15:951–960. - PubMed
-
- Baukrowitz T, Yellen G. Use-dependent blockers and exit rate of the last ion from the multi-ion pore of a K+channel. Science. 1996;271:653–656. - PubMed
-
- Bretag AH, Fietz MJ, Bennet RRJ. The effects of zinc and other transition metal ions on rat skeletal muscle. Proc Aust Physiol Pharmacol Soc. 1984;15:146P. . (Abstr.)

