Inhibition of duck hepatitis B virus replication by 9-(2-phosphonylmethoxyethyl)adenine, an acyclic phosphonate nucleoside analogue
- PMID: 9835503
- PMCID: PMC106011
- DOI: 10.1128/AAC.42.12.3130
Inhibition of duck hepatitis B virus replication by 9-(2-phosphonylmethoxyethyl)adenine, an acyclic phosphonate nucleoside analogue
Abstract
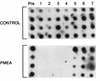
The use of regimens that use nucleoside analogues for the treatment of chronic hepatitis B virus infection is often limited because of their high relapse rates. This is thought to be due to the persistence of virus in nonhepatocyte reservoirs and/or the viral covalently closed circular (CCC) DNA species in the nucleus of infected hepatocytes. We have evaluated the novel nucleoside analogue 9-(2-phosphonylmethoxyethyl)adenine (PMEA) in the duck model of hepatitis B. Eight Pekin-Aylesbury ducks congenitally infected with the duck hepatitis B virus (DHBV) were treated with PMEA at a dosage of 15 mg/kg of body weight/day via the intraperitoneal route for 4 weeks. At the end of the treatment period, four animals were killed and the remainder were monitored for a further 4-week drug-free period before analysis. The results were compared with those for eight age-matched, untreated controls. The levels of viremia, the total intrahepatic DHBV load, and CCC DNA, viral RNA, and protein levels were measured by Southern hybridization, Northern hybridization, and immunoblotting of the appropriate specimen, respectively. Viral proteins and DNA were also measured by immunohistochemistry (IHC) and in situ hybridization (ISH) of sections of liver and pancreatic tissue. PMEA treatment reduced the viremia to undetectable levels, while the total viral DNA load in the liver was reduced by 95% compared to the control level. Viral RNA and protein levels decreased by approximately 30%. ISH and IHC confirmed the PMEA-related intrahepatic changes and established that the amount of virus in bile duct epithelial cells (BDEC) was reduced by 70% during therapy. During the follow-up period all parameters of active virological replication returned to those for the age-matched controls. PMEA had no significant effect upon the number of virus-infected islet or acinar cells in the pancreas. PMEA at a dosage of 15 mg/kg/day has potent activity against DHBV found within hepatocytes and BDEC and inhibits DHBV replication in BDEC.
Figures




Similar articles
-
Effect of nucleoside analogue therapy on duck hepatitis B viral replication in hepatocytes and bile duct epithelial cells in vivo.J Gastroenterol Hepatol. 2000 Mar;15(3):304-10. doi: 10.1046/j.1440-1746.2000.02079.x. J Gastroenterol Hepatol. 2000. PMID: 10764033
-
Inhibitory effect of 9-(2-phosphonylmethoxyethyl)-adenine (PMEA) on human and duck hepatitis B virus infection.Antiviral Res. 1993 Jun;21(2):141-53. doi: 10.1016/0166-3542(93)90050-s. Antiviral Res. 1993. PMID: 8338351
-
Inhibitory effect of adefovir on viral DNA synthesis and covalently closed circular DNA formation in duck hepatitis B virus-infected hepatocytes in vivo and in vitro.Antimicrob Agents Chemother. 2002 Feb;46(2):425-33. doi: 10.1128/AAC.46.2.425-433.2002. Antimicrob Agents Chemother. 2002. PMID: 11796353 Free PMC article.
-
In vitro antihepadnaviral activities of combinations of penciclovir, lamivudine, and adefovir.Antimicrob Agents Chemother. 2000 Mar;44(3):551-60. doi: 10.1128/AAC.44.3.551-560.2000. Antimicrob Agents Chemother. 2000. PMID: 10681317 Free PMC article.
-
The guanine nucleoside analog penciclovir is active against chronic duck hepatitis B virus infection in vivo.Antimicrob Agents Chemother. 1996 Feb;40(2):413-18. doi: 10.1128/AAC.40.2.413. Antimicrob Agents Chemother. 1996. PMID: 8834889 Free PMC article.
Cited by
-
Usefulness of serum HBV RNA levels for predicting antiviral response to entecavir treatment in patients with chronic hepatitis B.J Gastroenterol. 2025 Apr;60(4):469-478. doi: 10.1007/s00535-025-02211-5. Epub 2025 Jan 22. J Gastroenterol. 2025. PMID: 39841247 Free PMC article.
-
Antiviral efficacy and pharmacokinetics of oral adefovir dipivoxil in chronically woodchuck hepatitis virus-infected woodchucks.Antimicrob Agents Chemother. 2001 Oct;45(10):2740-5. doi: 10.1128/AAC.45.10.2740-2745.2001. Antimicrob Agents Chemother. 2001. PMID: 11557463 Free PMC article.
-
Carrier-mediated delivery of 9-(2-phosphonylmethoxyethyl)adenine to parenchymal liver cells: a novel therapeutic approach for hepatitis B.Antimicrob Agents Chemother. 2000 Mar;44(3):477-83. doi: 10.1128/AAC.44.3.477-483.2000. Antimicrob Agents Chemother. 2000. PMID: 10681306 Free PMC article.
-
Therapeutic Antiviral Effect of the Nucleic Acid Polymer REP 2055 against Persistent Duck Hepatitis B Virus Infection.PLoS One. 2015 Nov 11;10(11):e0140909. doi: 10.1371/journal.pone.0140909. eCollection 2015. PLoS One. 2015. PMID: 26560490 Free PMC article.
-
High-Density Lipoproteins: Nature's Multifunctional Nanoparticles.ACS Nano. 2016 Mar 22;10(3):3015-41. doi: 10.1021/acsnano.5b07522. Epub 2016 Feb 25. ACS Nano. 2016. PMID: 26889958 Free PMC article. Review.
References
-
- Aye T, Bartholomeusz A, Shaw T, Bowden D, Breschkin A, McMillan J, Angus P, Locarnini S. Hepatitis B virus polymerase mutations during antiviral therapy in a patient following liver transplantation. J Hepatol. 1997;26:1148–1153. - PubMed
-
- Beasley R P, Hwang L Y, Lin C C, Chien C S. Hepatocellular carcinoma and hepatitis B virus. A prospective study of 22,707 men in Taiwan. Lancet. 1981;ii:1129–1133. - PubMed
-
- Bishop N, Civitico G, Wang Y Y, Guo K, Birch C, Gust I, Locarnini S A. Antiviral strategies in chronic hepatitis B virus infection. I. Establishment of an in vitro system using the duck hepatitis B virus model. J Med Virol. 1990;3:82–89. - PubMed
-
- Boker K H W, Ringe B, Kruger M, Pichlmayr R, Manns M P. Prostaglandin E plus famciclovir—a new concept for the treatment of severe hepatitis B after liver transplantation. Transplantation. 1994;57:1706–1708. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

