ABT-378, a highly potent inhibitor of the human immunodeficiency virus protease
- PMID: 9835517
- PMCID: PMC106025
- DOI: 10.1128/AAC.42.12.3218
ABT-378, a highly potent inhibitor of the human immunodeficiency virus protease
Abstract
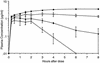
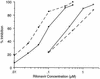
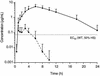
The valine at position 82 (Val 82) in the active site of the human immunodeficiency virus (HIV) protease mutates in response to therapy with the protease inhibitor ritonavir. By using the X-ray crystal structure of the complex of HIV protease and ritonavir, the potent protease inhibitor ABT-378, which has a diminished interaction with Val 82, was designed. ABT-378 potently inhibited wild-type and mutant HIV protease (Ki = 1.3 to 3.6 pM), blocked the replication of laboratory and clinical strains of HIV type 1 (50% effective concentration [EC50], 0.006 to 0.017 microM), and maintained high potency against mutant HIV selected by ritonavir in vivo (EC50, </=0. 06 microM). The metabolism of ABT-378 was strongly inhibited by ritonavir in vitro. Consequently, following concomitant oral administration of ABT-378 and ritonavir, the concentrations of ABT-378 in rat, dog, and monkey plasma exceeded the in vitro antiviral EC50 in the presence of human serum by >50-fold after 8 h. In healthy human volunteers, coadministration of a single 400-mg dose of ABT-378 with 50 mg of ritonavir enhanced the area under the concentration curve of ABT-378 in plasma by 77-fold over that observed after dosing with ABT-378 alone, and mean concentrations of ABT-378 exceeded the EC50 for >24 h. These results demonstrate the potential utility of ABT-378 as a therapeutic intervention against AIDS.
Figures

References
-
- Cameron D W, Heath-Chiozzi M, Danner S, Cohen C, Krabcik S, Maurath C, Sun E, Henry D, Rode R, Potoff A, Leonard J. Randomised placebo-controlled trial of ritonavir in advanced HIV-1 disease. Lancet. 1998;351:543–549. - PubMed
-
- Clumeck N. Program and abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy, Toronto, Canada. Washington, D.C: American Society for Microbiology; 1997. Clinical benefit of saquinavir (SQV) plus zalcitabine (ddC) plus zidovudine (ZDV) in untreated/minimally treated HIV-infected patients, abstr. LB-4.
-
- Danner S A, Carr A, Leonard J M, Lehman L M, Gudiol F, Gonzales J, Raventos A, Rubio R, Bouza E, Pintado V, Gil Aguado A, Garcia de Lomas J, Delgado R, Borleffs J C C, Hsu A, Valdes J, Boucher C A B, Cooper D A. A short-term study of the safety, pharmacokinetics, and efficacy of ritonavir, an inhibitor of HIV-1 protease. N Engl J Med. 1995;333:1528–1533. - PubMed
-
- Greco W R, Hakala M T. Evaluation of methods for estimating the dissociation constant of tight binding inhibitors. J Biol Chem. 1979;254:12104–12109. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

