Induction of resistance to azole drugs in Trypanosoma cruzi
- PMID: 9835521
- PMCID: PMC106029
- DOI: 10.1128/AAC.42.12.3245
Induction of resistance to azole drugs in Trypanosoma cruzi
Abstract
Trypanosoma cruzi is the protozoan parasite that causes Chagas' disease, a frequently fatal illness affecting the heart and gastrointestinal systems. An estimated 16 million to 18 million people in Latin America and 50,000 to 100,000 people in the United States are infected with this pathogen. Treatment options for T. cruzi infections are suboptimal due to the toxicities and limited effectiveness of the available drugs. Azole antimicrobial agents have been discovered to have antitrypanosomal activity by inhibition of ergosterol synthesis. The triazole itraconazole was recently shown to produce a parasitologic cure rate of 53% in chronically infected patients (W. Apt et al., Am. J. Trop. Med. Hyg. 59:133-138, 1998), a result which may lead to more use of this family of drugs for the treatment of T. cruzi infections. In the experiments reported on here, resistance to azoles was induced in vitro by serial passage of mammalian-stage parasites in the presence of fluconazole for 4 months. These parasites were cross resistant to the other azoles, ketoconazole, miconazole, and itraconazole. They remained susceptible to benznidazole and amphotericin B. The azole-resistant phenotype was stable for more than 2 months of in vitro serial passage without fluconazole. In addition, the parasites resisted treatment in mice receiving ketoconazole. The rapid development of azole resistance in T. cruzi in vitro suggests that resistance to azole drugs has the potential to occur in patients and may pose an impediment to the progress being made in the treatment of T. cruzi infection.
Figures
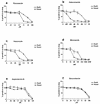

 ) and ketoconazole-treated
(
) and ketoconazole-treated
( ) mice infected with the parent
line on all 3 days (P < 0.05), whereas the levels of
parasitemia were not significantly different between the
placebo-treated (▩) and ketoconazole-treated (■) mice infected with
the azole-resistant T. cruzi strain on all 3 days
(P > 0.10). hpf, high-power field.
) mice infected with the parent
line on all 3 days (P < 0.05), whereas the levels of
parasitemia were not significantly different between the
placebo-treated (▩) and ketoconazole-treated (■) mice infected with
the azole-resistant T. cruzi strain on all 3 days
(P > 0.10). hpf, high-power field.Similar articles
-
Glutamine metabolism modulates azole susceptibility in Trypanosoma cruzi amastigotes.Elife. 2020 Dec 1;9:e60226. doi: 10.7554/eLife.60226. Elife. 2020. PMID: 33258448 Free PMC article.
-
[The chemotherapy of Chagas disease].Medicina (B Aires). 1999;59 Suppl 2:147-65. Medicina (B Aires). 1999. PMID: 10668258 Review. Spanish.
-
In vivo selection of a population of Trypanosoma cruzi and clones resistant to benznidazole.Parasitology. 1998 Feb;116 ( Pt 2):165-71. doi: 10.1017/s0031182097002084. Parasitology. 1998. PMID: 9509026
-
Mevinolin (lovastatin) potentiates the antiproliferative effects of ketoconazole and terbinafine against Trypanosoma (Schizotrypanum) cruzi: in vitro and in vivo studies.Antimicrob Agents Chemother. 1993 Mar;37(3):580-91. doi: 10.1128/AAC.37.3.580. Antimicrob Agents Chemother. 1993. PMID: 8460926 Free PMC article.
-
Sterol 14-demethylase inhibitors for Trypanosoma cruzi infections.Adv Exp Med Biol. 2008;625:61-80. doi: 10.1007/978-0-387-77570-8_6. Adv Exp Med Biol. 2008. PMID: 18365659 Review.
Cited by
-
A nonazole CYP51 inhibitor cures Chagas' disease in a mouse model of acute infection.Antimicrob Agents Chemother. 2010 Jun;54(6):2480-8. doi: 10.1128/AAC.00281-10. Epub 2010 Apr 12. Antimicrob Agents Chemother. 2010. PMID: 20385875 Free PMC article.
-
3-pyridyl inhibitors with novel activity against Trypanosoma cruzi reveal in vitro profiles can aid prediction of putative cytochrome P450 inhibition.Sci Rep. 2018 Mar 20;8(1):4901. doi: 10.1038/s41598-018-22043-z. Sci Rep. 2018. PMID: 29559688 Free PMC article.
-
Gene expression study using real-time PCR identifies an NTR gene as a major marker of resistance to benzonidazole in Trypanosoma cruzi.Parasit Vectors. 2011 Sep 5;4:169. doi: 10.1186/1756-3305-4-169. Parasit Vectors. 2011. PMID: 21892937 Free PMC article.
-
Nitroheterocyclic compounds are more efficacious than CYP51 inhibitors against Trypanosoma cruzi: implications for Chagas disease drug discovery and development.Sci Rep. 2014 Apr 16;4:4703. doi: 10.1038/srep04703. Sci Rep. 2014. PMID: 24736467 Free PMC article.
-
Structures of prostaglandin F synthase from the protozoa Leishmania major and Trypanosoma cruzi with NADP.Acta Crystallogr F Struct Biol Commun. 2015 May;71(Pt 5):609-14. doi: 10.1107/S2053230X15006883. Epub 2015 Apr 21. Acta Crystallogr F Struct Biol Commun. 2015. PMID: 25945716 Free PMC article.
References
-
- Ajioka J, Swindle J. The calmodulin-ubiquitin (CUB) genes of Trypanosoma cruziare essential for parasite viability. Mol Biochem Parasitol. 1996;78:217–225. - PubMed
-
- Apt W, Aguilera X, Arribada A, Perez C, Miranda C, Sanchez G, Zulantay I, Cortes P, Rodriguez J, Juri D. Treatment of chronic Chagas’ disease with itraconazole and allopurinol. Am J Trop Med Hyg. 1998;59:133–138. - PubMed
-
- Bacchi C J. Resistance to clinical drugs in African trypanosomes. Parasitol Today. 1993;9:190–193. - PubMed
-
- Beverley S M. Gene amplification in Leishmania. Annu Rev Microbiol. 1991;45:417–444. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

