Procaryotic expression of single-chain variable-fragment (scFv) antibodies: secretion in L-form cells of Proteus mirabilis leads to active product and overcomes the limitations of periplasmic expression in Escherichia coli
- PMID: 9835575
- PMCID: PMC90935
- DOI: 10.1128/AEM.64.12.4862-4869.1998
Procaryotic expression of single-chain variable-fragment (scFv) antibodies: secretion in L-form cells of Proteus mirabilis leads to active product and overcomes the limitations of periplasmic expression in Escherichia coli
Abstract
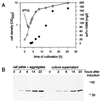
Recently it has been demonstrated that L-form cells of Proteus mirabilis (L VI), which lack a periplasmic compartment, can be efficiently used in the production and secretion of heterologous proteins. In search of novel expression systems for recombinant antibodies, we compared levels of single-chain variable-fragment (scFv) production in Escherichia coli JM109 and P. mirabilis L VI, which express four distinct scFvs of potential clinical interest that show differences in levels of expression and in their tendencies to form aggregates upon periplasmic expression. Production of all analyzed scFvs in E. coli was limited by the severe toxic effect of the heterologous product as indicated by inhibition of culture growth and the formation of insoluble aggregates in the periplasmic space, limiting the yield of active product. In contrast, the L-form cells exhibited nearly unlimited growth under the tested production conditions for all scFvs examined. Moreover, expression experiments with P. mirabilis L VI led to scFv concentrations in the range of 40 to 200 mg per liter of culture medium (corresponding to volume yields 33- to 160-fold higher than those with E. coli JM109), depending on the expressed antibody. In a translocation inhibition experiment the secretion of the scFv constructs was shown to be an active transport coupled to the signal cleavage. We suppose that this direct release of the newly synthesized product into a large volume of the growth medium favors folding into the native active structure. The limited aggregation of scFv observed in the P. mirabilis L VI supernatant (occurring in a first-order-kinetics manner) was found to be due to intrinsic features of the scFv and not related to the expression process of the host cells. The P. mirabilis L VI supernatant was found to be advantageous for scFv purification. A two-step chromatography procedure led to homogeneous scFv with high antigen binding activity as revealed from binding experiments with eukaryotic cells.
Figures






Similar articles
-
High cytoplasmic expression in E. coli, purification, and in vitro refolding of a single chain Fv antibody fragment against the hepatitis B surface antigen.J Biotechnol. 1999 Jun 11;72(1-2):13-20. doi: 10.1016/s0168-1656(99)00036-x. J Biotechnol. 1999. PMID: 10406095
-
Production, purification, and characterization of human scFv antibodies expressed in Saccharomyces cerevisiae, Pichia pastoris, and Escherichia coli.Protein Expr Purif. 2005 Aug;42(2):255-67. doi: 10.1016/j.pep.2005.04.015. Protein Expr Purif. 2005. PMID: 15946857
-
A simple vector system to improve performance and utilisation of recombinant antibodies.BMC Biotechnol. 2006 Dec 7;6:46. doi: 10.1186/1472-6750-6-46. BMC Biotechnol. 2006. PMID: 17156422 Free PMC article.
-
Production of phenylpyruvic acid by engineered L-amino acid deaminase from Proteus mirabilis.Biotechnol Lett. 2022 Jun;44(5-6):635-642. doi: 10.1007/s10529-022-03245-y. Epub 2022 Apr 16. Biotechnol Lett. 2022. PMID: 35429303 Review.
-
Secretion of recombinant proteins from E. coli.Eng Life Sci. 2018 Apr 14;18(8):532-550. doi: 10.1002/elsc.201700200. eCollection 2018 Aug. Eng Life Sci. 2018. PMID: 32624934 Free PMC article. Review.
Cited by
-
Expression of recombinant antibodies.Front Immunol. 2013 Jul 29;4:217. doi: 10.3389/fimmu.2013.00217. eCollection 2013. Front Immunol. 2013. PMID: 23908655 Free PMC article.
-
Osmotic conditions could promote scFv antibody production in the Escherichia coli HB2151.Bioimpacts. 2017;7(3):199-206. doi: 10.15171/bi.2017.23. Epub 2017 Aug 23. Bioimpacts. 2017. PMID: 29159147 Free PMC article.
-
A next-generation Fab library platform directly yielding drug-like antibodies with high affinity, diversity, and developability.MAbs. 2024 Jan-Dec;16(1):2394230. doi: 10.1080/19420862.2024.2394230. Epub 2024 Aug 27. MAbs. 2024. PMID: 39192463 Free PMC article.
-
The production of antibody fragments and antibody fusion proteins by yeasts and filamentous fungi.Microb Cell Fact. 2003 Jan 30;2(1):1. doi: 10.1186/1475-2859-2-1. Microb Cell Fact. 2003. PMID: 12605725 Free PMC article.
-
Biofilm architecture: An emerging synthetic biology target.Synth Syst Biotechnol. 2020 Jan 13;5(1):1-10. doi: 10.1016/j.synbio.2020.01.001. eCollection 2020 Mar. Synth Syst Biotechnol. 2020. PMID: 31956705 Free PMC article. Review.
References
-
- Bowden G A, Georgiou G. Folding and aggregation of β-lactamase in the periplasmic space of E. coli. J Biol Chem. 1990;265:16760–16766. - PubMed
-
- Brocks B, Rode H-J, Klein M, Gerlach E, Dübel S, Little M, Pfizenmaier K, Moosmayer D. A TNF receptor antagonist scFv, which is not secreted in mammalian cells, is expressed as a soluble mono- and bivalent scFv derivative in insect cells. Immunotechnology (Amsterdam) 1997;4:173–184. - PubMed
-
- Driessen A J M. How proteins cross the bacterial cytoplasmic membrane. J Membr Biol. 1994;142:145–159. - PubMed
-
- Dübel S, Breitling F, Klewinghaus I, Little M. Regulated secretion and purification of recombinant antibodies in E. coli. Cell Biophys. 1992;21:69–79. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

