Sinorhizobium fredii and Sinorhizobium meliloti produce structurally conserved lipopolysaccharides and strain-specific K antigens
- PMID: 9835585
- PMCID: PMC90945
- DOI: 10.1128/AEM.64.12.4930-4938.1998
Sinorhizobium fredii and Sinorhizobium meliloti produce structurally conserved lipopolysaccharides and strain-specific K antigens
Abstract
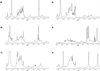
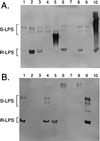
Lipopolysaccharides (LPS) and capsular polysaccharides (K antigens) may influence the interaction of rhizobia with their specific hosts; therefore, we conducted a comparative analysis of Sinorhizobium fredii and Sinorhizobium meliloti, which are genetically related, yet symbiotically distinct, nitrogen-fixing microsymbionts of legumes. We found that both species typically produce strain-specific K antigens that consist of 3-deoxy-D-manno-2-octulosonic acid (Kdo), or other 1-carboxy-2-keto-3-deoxy sugars (such as sialic acid), and hexoses. The K antigens of each strain are distinguished by glycosyl composition, anomeric configuration, acetylation, and molecular weight distribution. One consistent difference between the K antigens of S. fredii and those of S. meliloti is the presence of N-acetyl groups in the polysaccharides of the latter. In contrast to the K antigens, the LPS of Sinorhizobium spp. are major common antigens. Rough (R) LPS is the predominant form of LPS produced by cultured cells, and some strains release almost no detectable smooth (S) LPS upon extraction. Sinorhizobium spp. are delineated into two major RLPS core serogroups, which do not correspond to species (i.e., host range). The O antigens of the SLPS, when present, have similar degrees of polymerization and appear to be structurally conserved throughout the genus. Interestingly, one strain was found to be distinct from all others: S. fredii HH303 produces a unique K antigen, which contains galacturonic acid and rhamnose, and the RLPS did not fall into either of the RLPS core serogroups. The results of this study indicate that the conserved S- and RLPS of Sinorhizobium spp. lack the structural information necessary to influence host specificity, whereas the variable K antigens may affect strain-cultivar interactions.
Figures
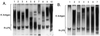


References
-
- Bequart-de Kozak I, Reuhs B L, Buffard D, Breda C, Kim J S, Esnault R, Kondorosi A. Role of the K-antigen subgroup of capsular polysaccharides in the early recognition process between Rhizobium meliloti and alfalfa leaves. Mol Plant-Microbe Interact. 1997;10:114–123.
-
- Carlson R W, Bhat U R, Reuhs B. Rhizobium lipopolysaccharides: their structures and evidence for their importance in the nitrogen-fixing symbiotic infection of their host legumes. In: Gresshoff P M, editor. Plant biotechnology and development. Boca Raton, Fla: CRC Press; 1992. pp. 33–44.
-
- Carlson R W, Krishnaiah B S. Structures of the oligosaccharides obtained from the core regions of the lipopolysaccharides of Bradyrhizobium japonicum 61A101c and its symbiotically defective lipopolysaccharide mutant, JS314. Carbohydr Res. 1992;231:205–219. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

