Concurrent administration of donepezil HCl and digoxin: assessment of pharmacokinetic changes
- PMID: 9839765
- PMCID: PMC1873815
- DOI: 10.1046/j.1365-2125.1998.0460s1040.x
Concurrent administration of donepezil HCl and digoxin: assessment of pharmacokinetic changes
Abstract
Aim: The aim of this study was to examine the pharmacokinetics of donepezil HCl and digoxin separately, and in combination, following administration of single oral doses. Changes in cardiac conduction parameters following drug administration were also assessed.
Methods: This was an open-label, randomized, three-period crossover study in healthy male volunteers (n=12). During each treatment period, subjects received a single dose of either donepezil HCl (5 mg), digoxin (0.25 mg), or a combination of both drugs. Each treatment period was followed by a 2-week, drug-free washout period.
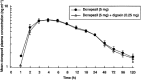
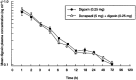
Results: All 12 volunteers completed the study without incident. No statistically significant differences in donepezil pharmacokinetics (Cmax, tmax, AUC(0-120), AUC(0-infinity) or t1/2) were observed when donepezil administered alone was compared with donepezil administered in combination with digoxin. Similarly, no statistically significant differences in digoxin pharmacokinetics were observed when digoxin was administered alone or in combination with donepezil. No clinically relevant changes in cardiac conduction (lead II ECG) were observed in any subject during any treatment period.
Conclusions: Co-administration of single doses of donepezil HCl (5 mg) and digoxin (0.25 mg) produced no changes in the pharmacokinetic profile of either drug. In addition, co-administration produced no changes in cardiac conduction parameters during the 24 h of telemetry monitoring following drug administration.
Figures
References
-
- Katzman R, Saitoh T. Advances in Alzheimer’s disease. FASEB J. 1991;5:278–286. - PubMed
-
- Becker RE. Therapy of the cognitive deficit in Alzheimer’s disease: the cholinergic system. In: Becker R, Giacobini E, editors. Cholinergic Basis for Alzheimer Therapy. Boston: Birkhäuser; 1991. pp. 1–30.
-
- Giacobini E. Pharmacotherapy of AD: new drugs and novel strategies. In: Corain B, Iqbal K, Nicolini M, Winblad B, Wisniewski HM, Zatta PF, editors. Alzheimer’s Disease—Advances in Clinical and Basic Research. New York: Wiley; 1993. pp. 529–538.
-
- Giacobini E. Therapy for Alzheimer’s disease: symptomatic or neuroprotective? Mol Neurol. 1994;9:115–117. - PubMed
-
- Small DH, Michaelson S. Do cholinesterases have functions unrelated to neurotransmission? Today’s Life Sci. 1995;7:24–30.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources