Multimodular penicillin-binding proteins: an enigmatic family of orthologs and paralogs
- PMID: 9841666
- PMCID: PMC98940
- DOI: 10.1128/MMBR.62.4.1079-1093.1998
Multimodular penicillin-binding proteins: an enigmatic family of orthologs and paralogs
Abstract
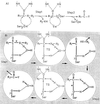
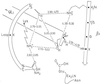
The monofunctional penicillin-binding DD-peptidases and penicillin-hydrolyzing serine beta-lactamases diverged from a common ancestor by the acquisition of structural changes in the polypeptide chain while retaining the same folding, three-motif amino acid sequence signature, serine-assisted catalytic mechanism, and active-site topology. Fusion events gave rise to multimodular penicillin-binding proteins (PBPs). The acyl serine transferase penicillin-binding (PB) module possesses the three active-site defining motifs of the superfamily; it is linked to the carboxy end of a non-penicillin-binding (n-PB) module through a conserved fusion site; the two modules form a single polypeptide chain which folds on the exterior of the plasma membrane and is anchored by a transmembrane spanner; and the full-size PBPs cluster into two classes, A and B. In the class A PBPs, the n-PB modules are a continuum of diverging sequences; they possess a five-motif amino acid sequence signature, and conserved dicarboxylic amino acid residues are probably elements of the glycosyl transferase catalytic center. The PB modules fall into five subclasses: A1 and A2 in gram-negative bacteria and A3, A4, and A5 in gram-positive bacteria. The full-size class A PBPs combine the required enzymatic activities for peptidoglycan assembly from lipid-transported disaccharide-peptide units and almost certainly prescribe different, PB-module specific traits in peptidoglycan cross-linking. In the class B PBPs, the PB and n-PB modules cluster in a concerted manner. A PB module of subclass B2 or B3 is linked to an n-PB module of subclass B2 or B3 in gram-negative bacteria, and a PB module of subclass B1, B4, or B5 is linked to an n-PB module of subclass B1, B4, or B5 in gram-positive bacteria. Class B PBPs are involved in cell morphogenesis. The three motifs borne by the n-PB modules are probably sites for module-module interaction and the polypeptide stretches which extend between motifs 1 and 2 are sites for protein-protein interaction. The full-size class B PBPs are an assortment of orthologs and paralogs, which prescribe traits as complex as wall expansion and septum formation. PBPs of subclass B1 are unique to gram-positive bacteria. They are not essential, but they represent an important mechanism of resistance to penicillin among the enterococci and staphylococci. Natural evolution and PBP- and beta-lactamase-mediated resistance show that the ability of the catalytic centers to adapt their properties to new situations is limitless. Studies of the reaction pathways by using the methods of quantum chemistry suggest that resistance to penicillin is a road of no return.
Figures


References
-
- Adam M, Fraipont C, Rhazi N, Nguyen-Distèche M, Lakaye B, Frère J M, Devreese B, Van Beeumen J, van Heijenoort Y, van Heijenoort J, Ghuysen J M. The bimodular G57-V577 polypeptide chain of the class B penicillin-binding protein 3 of Escherichia coli catalyses peptide bond formation from thiolesters and does not catalyze glycan chain polymerization from lipid II intermediate. J Bacteriol. 1997;179:6005–6009. - PMC - PubMed
-
- Barton G J. Protein multiple sequence alignment and flexible pattern matching. Methods Enzymol. 1990;183:403–428. - PubMed
-
- Basu J, Mahapatra S, Kundu M, Mukhopadhyay S, Nguyen-Distèche M, Dubois P, Joris B, Van Beeumen J, Cole S T, Chakrabarti P, Ghuysen J M. Identification and overexpression in Escherichia coli of a Mycobacterium leprae gene, pon1, encoding high-molecular-mass class A penicillin-binding protein PBP1. J Bacteriol. 1996;178:1707–1711. - PMC - PubMed
-
- Berger-Bächi B. Expression of resistance to methicillin. Trends Microbiol. 1994;2:389–393. - PubMed
-
- Blattner F R, et al. The complete genome sequence of Escherichia coli K12. Science. 1997;277:1453–1462. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

