The virulence plasmid of Yersinia, an antihost genome
- PMID: 9841674
- PMCID: PMC98948
- DOI: 10.1128/MMBR.62.4.1315-1352.1998
The virulence plasmid of Yersinia, an antihost genome
Abstract
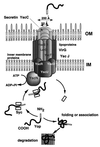
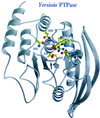
The 70-kb virulence plasmid enables Yersinia spp. (Yersinia pestis, Y. pseudotuberculosis, and Y. enterocolitica) to survive and multiply in the lymphoid tissues of their host. It encodes the Yop virulon, an integrated system allowing extracellular bacteria to disarm the cells involved in the immune response, to disrupt their communications, or even to induce their apoptosis by the injection of bacterial effector proteins. This system consists of the Yop proteins and their dedicated type III secretion apparatus, called Ysc. The Ysc apparatus is composed of some 25 proteins including a secretin. Most of the Yops fall into two groups. Some of them are the intracellular effectors (YopE, YopH, YpkA/YopO, YopP/YopJ, YopM, and YopT), while the others (YopB, YopD, and LcrV) form the translocation apparatus that is deployed at the bacterial surface to deliver the effectors into the eukaryotic cells, across their plasma membrane. Yop secretion is triggered by contact with eukaryotic cells and controlled by proteins of the virulon including YopN, TyeA, and LcrG, which are thought to form a plug complex closing the bacterial secretion channel. The proper operation of the system also requires small individual chaperones, called the Syc proteins, in the bacterial cytosol. Transcription of the genes is controlled both by temperature and by the activity of the secretion apparatus. The virulence plasmid of Y. enterocolitica and Y. pseudotuberculosis also encodes the adhesin YadA. The virulence plasmid contains some evolutionary remnants including, in Y. enterocolitica, an operon encoding resistance to arsenic compounds.
Figures


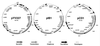



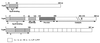

References
-
- Alfano J R, Kim H S, Delaney T P, Collmer A. Evidence that the Pseudomonas syringae pv. syringae hrp-linked hrmA gene encodes an Avr-like protein that acts in an hrp-dependent manner within tobacco cells. Mol Plant-Microbe Interact. 1997;10:580–588. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

