Staphylococcal cell wall: morphogenesis and fatal variations in the presence of penicillin
- PMID: 9841676
- PMCID: PMC98950
- DOI: 10.1128/MMBR.62.4.1371-1414.1998
Staphylococcal cell wall: morphogenesis and fatal variations in the presence of penicillin
Abstract
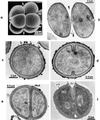
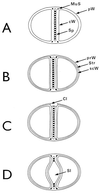
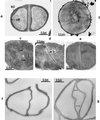
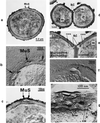
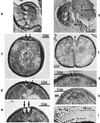
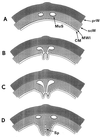
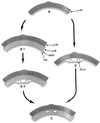
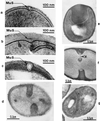
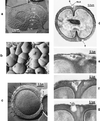
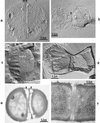
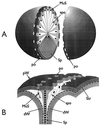
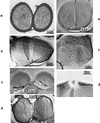
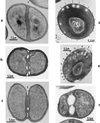
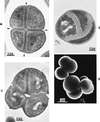
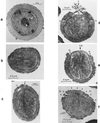
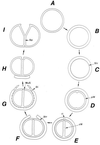
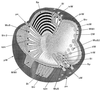
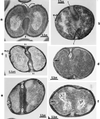
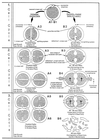
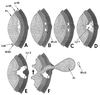
The primary goal of this review is to provide a compilation of the complex architectural features of staphylococcal cell walls and of some of their unusual morphogenetic traits including the utilization of murosomes and two different mechanisms of cell separation. Knowledge of these electron microscopic findings may serve as a prerequisite for a better understanding of the sophisticated events which lead to penicillin-induced death. For more than 50 years there have been controversial disputes about the mechanisms by which penicillin kills bacteria. Many hypotheses have tried to explain this fatal event biochemically and mainly via bacteriolysis. However, indications that penicillin-induced death of staphylococci results from overall biochemical defects or from a fatal attack of bacterial cell walls by bacteriolytic murein hydrolases were not been found. Rather, penicillin, claimed to trigger the activity of murein hydrolases, impaired autolytic wall enzymes of staphylococci. Electron microscopic investigations have meanwhile shown that penicillin-mediated induction of seemingly minute cross wall mistakes is the very reason for this killing. Such "morphogenetic death" taking place at predictable cross wall sites and at a predictable time is based on the initiation of normal cell separations in those staphylococci in which the completion of cross walls had been prevented by local penicillin-mediated impairment of the distribution of newly synthesized peptidoglycan; this death occurs because the high internal pressure of the protoplast abruptly kills such cells via ejection of some cytoplasm during attempted cell separation. An analogous fatal onset of cell partition is considered to take place without involvement of a detectable quantity of autolytic wall enzymes ("mechanical cell separation"). The most prominent feature of penicillin, the disintegration of bacterial cells via bacteriolysis, is shown to represent only a postmortem process resulting from shrinkage of dead cells and perturbation of the cytoplasmic membrane. Several schematic drawings have been included in this review to facilitate an understanding of the complex morphogenetic events.
Figures


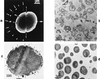
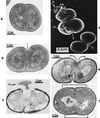

References
-
- Abraham E P. From penicillins to cephalosporins. In: Kleinkauf H, von Döhren H, editors. 50 years of penicillin application—history and trends. Berlin, Germany: Technische Universität Berlin; 1993. pp. 7–23.
-
- Abraham E P, Fletcher C M, Florey H W, Gardner A D, Heatley N G, Jennings M A. Further observations on penicillin. Lancet. 1941;ii:177–189. - PubMed
-
- Amako K, Umeda A. Scanning electron microscopy of Staphylococcus. J Ultrastruct Res. 1977;58:34–40. - PubMed
-
- Amako K, Umeda A. Concentric circular structure of the cell wall of Staphylococcus epidermidis revealed by scanning electron microscope. J Electron Microsc. 1978;27:147–148. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

