2B4, the natural killer and T cell immunoglobulin superfamily surface protein, is a ligand for CD48
- PMID: 9841922
- PMCID: PMC2212392
- DOI: 10.1084/jem.188.11.2083
2B4, the natural killer and T cell immunoglobulin superfamily surface protein, is a ligand for CD48
Abstract
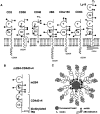
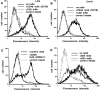
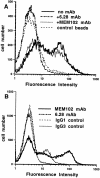
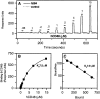
2B4 is a cell surface glycoprotein related to CD2 and implicated in the regulation of natural killer and T lymphocyte function. A recombinant protein containing the extracellular region of mouse (m)2B4 attached to avidin-coated fluorescent beads bound to rodent cells, and binding was completely blocked by CD48 monoclonal antibodies (mAbs). Using surface plasmon resonance, we showed that purified soluble mCD48 bound m2B4 with a six- to ninefold higher affinity (Kd approximately 16 microM at 37 degreesC) than its other ligand, CD2. Human CD48 bound human 2B4 with a similar affinity (Kd approximately 8 microM). The finding of an additional ligand for CD48 provides an explanation for distinct functional effects observed on perturbing CD2 and CD48 with mAbs or by genetic manipulation.
Figures



References
-
- Barclay, A.N., M.H. Brown, S.K.A. Law, A.J. McKnight, M.G. Tomlinson, and P.A. van der Merwe. 1997. The Leucocyte Antigen Factsbook. 2nd ed. Academic Press Ltd., London. 613 pp.
-
- Preston S, Wright GJ, Starr K, Barclay AN, Brown MH. The leukocyte/neuron cell surface antigen OX2 binds to a ligand on macrophages. Eur J Immunol. 1997;27:1911–1918. - PubMed
-
- Selvaraj P, Plunkett ML, Dustin M, Sanders ME, Shaw S, Springer TA. The T lymphocyte glycoprotein CD2 binds the cell surface ligand LFA-3. Nature. 1987;326:400–403. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

