Human toll-like receptor 2 confers responsiveness to bacterial lipopolysaccharide
- PMID: 9841923
- PMCID: PMC2212382
- DOI: 10.1084/jem.188.11.2091
Human toll-like receptor 2 confers responsiveness to bacterial lipopolysaccharide
Abstract
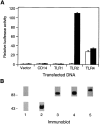
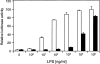
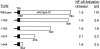
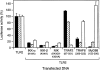
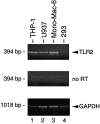
Bacterial lipopolysaccharide (LPS) induces activation of the transcription factor nuclear factor kappaB (NF-kappaB) in host cells upon infection. LPS binds to the glycosylphosphatidylinositol (GPI)- anchored membrane protein CD14, which lacks an intracellular signaling domain. Here we investigated the role of mammalian Toll-like receptors (TLRs) as signal transducers for LPS. Overexpression of TLR2, but not TLR1, TLR4, or CD14 conferred LPS inducibility of NF-kappaB activation in mammalian 293 cells. Mutational analysis demonstrated that this LPS response requires the intracellular domain of TLR2. LPS signaling through TLR2 was dependent on serum which contains soluble CD14 (sCD14). Coexpression of CD14 synergistically enhanced LPS signal transmission through TLR2. In addition, purified recombinant sCD14 could substitute for serum to support LPS-induced TLR2 activation. LPS stimulation of TLR2 initiated an interleukin 1 receptor-like NF-kappaB signaling cascade. These findings suggest that TLR2 may be a signaling component of a cellular receptor for LPS.
Figures



References
-
- Schletter J, Heine H, Ulmer AJ, Rietschel ET. Molecular mechanisms of endotoxin activity. Arch Microbiol. 1995;164:383–389. - PubMed
-
- Ulevitch RJ, Tobias PS. Receptor-dependent mechanisms of cell stimulation by bacterial endotoxin. Annu Rev Immunol. 1995;13:437–457. - PubMed
-
- Belvin MP, Anderson KV. A conserved signaling pathway: the Drosophilatoll-dorsal pathway. Annu Rev Cell Dev Biol. 1996;12:393–416. - PubMed
-
- Hoffmann JA, Reichhart J-M. Drosophilaimmunity. Trends Cell Biol. 1997;7:309–316. - PubMed
-
- Mitcham JL, Partnet P, Bonnert TP, Garka KE, Gerhart MJ, Slack JL, Gayle MA, Dower SK, Sims JE. T1/ST2 signaling establishes it as a member of an expanding interleukin-1 receptor family. J Biol Chem. 1996;271:5777–5783. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

