Yersinia-induced apoptosis in vivo aids in the establishment of a systemic infection of mice
- PMID: 9841926
- PMCID: PMC2212385
- DOI: 10.1084/jem.188.11.2127
Yersinia-induced apoptosis in vivo aids in the establishment of a systemic infection of mice
Abstract
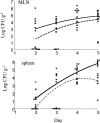
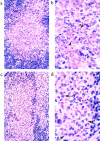
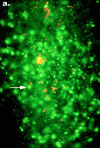
Pathogenic Yersinia cause a systemic infection in mice that is dependent on the presence of a large plasmid encoding a number of secreted virulence proteins called Yops. We previously demonstrated that a plasmid-encoded Yop, YopJ, was essential for inducing apoptosis in cultured macrophages. Here we report that YopJ is a virulence factor in mice and is important for the establishment of a systemic infection. The oral LD50 for a yopJ mutant Yersinia pseudotuberculosis increases 64-fold compared with wild-type. Although the yopJ mutant strain is able to reach the spleen of infected mice, the mutant strain seldom reaches the same high bacterial load that is seen with wild-type Yersinia strain and begins to be cleared from infected spleens on day 4 after infection. Furthermore, when in competition with wild-type Yersinia in a mixed infection, the yopJ mutant strain is deficient for spread from the Peyer's patches to other lymphoid tissue. We also show that wild-type Yersinia induces apoptosis in vivo of Mac-1(+) cells from infected mesenteric lymph nodes or spleens, as measured by quantitative flow cytometry of TUNEL (Tdt-mediated dUTP-biotin nick-end labeling)-positive cells. The levels of Mac-1(+), TUNEL+ cells from tissue infected with the yopJ mutant strain were equivalent to the levels detected in cells from uninfected tissue. YopJ is necessary for the suppression of TNF-alpha production seen in macrophages infected with wild-type Yersinia, based on previous in vitro studies (Palmer, L.E., S. Hobbie, J.E. Galan, and J.B. Bliska. 1998. Mol. Microbiol. 27:953-965). We conclude here that YopJ plays a role in the establishment of a systemic infection by inducing apoptosis and that this is consistent with the ability to suppress the production of the proinflammatory cytokine tumor necrosis factor alpha.
Figures


References
-
- Cornelis G, Laroche Y, Balligand G, Sory MP, Wauters G. Yersinia enterocolitica, a primary model for bacterial invasiveness. Rev Infect Dis. 1987;9:64–87. - PubMed
-
- Bottone EJ. Yersinia enterocolitica: a panoramic view of a charismatic microorganism. CRC Crit Rev Microbiol. 1977;5:211–241. - PubMed
-
- Ahvonen P, Sievers K, Aho K. Arthritis associated with Yersinia enterocoliticainfection. Acta Rheumatol Scand. 1969;15:232–253. - PubMed
-
- Bouza E, Dominguez A, Meseguer M, Buzon L, Boixeda D, Revillo MJ, de Rafael L, Martinez-Beltran J. Yersinia enterocoliticaSepticemia. Am J Clin Pathol. 1980;74:404–409. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

