Dendritic cell survival and maturation are regulated by different signaling pathways
- PMID: 9841930
- PMCID: PMC2212396
- DOI: 10.1084/jem.188.11.2175
Dendritic cell survival and maturation are regulated by different signaling pathways
Abstract
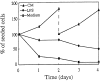
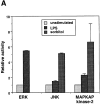
Although dendritic cell (DC) activation is a critical event for the induction of immune responses, the signaling pathways involved in this process have not been characterized. In this report, we show that DC activation induced by lipopolysaccharide (LPS) can be separated into two distinct processes: first, maturation, leading to upregulation of MHC and costimulatory molecules, and second, rescue from immediate apoptosis after withdrawal of growth factors (survival). Using a DC culture system that allowed us to propagate immature growth factor-dependent DCs, we have investigated the signaling pathways activated by LPS. We found that LPS induced nuclear translocation of the nuclear factor (NF)-kappaB transcription factor. Inhibition of NF-kappaB activation blocked maturation of DCs in terms of upregulation of major histocompatibility complex and costimulatory molecules. In addition, we found that LPS activated the extracellular signal-regulated kinase (ERK), and that specific inhibition of MEK1, the kinase which activates ERK, abrogated the ability of LPS to prevent apoptosis but did not inhibit DC maturation or NF-kappaB nuclear translocation. These results indicate that ERK and NF-kappaB regulate different aspects of LPS-induced DC activation: ERK regulates DC survival whereas NF-kappaB is responsible for DC maturation.
Figures



References
-
- Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature. 1998;392:245–251. - PubMed
-
- Cella M, Hengering A, Pinet V, Pieters J, Lanzavecchia A. Inflammatory stimuli induces accumulation of MHC class II complexes on dendritic cells. Nature. 1997;388:782–787. - PubMed
-
- May MJ, Ghosh S. Signal transduction through NF-kappa B. Immunol Today. 1998;19:80–88. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

