Apoprotein B100 has a prolonged interaction with the translocon during which its lipidation and translocation change from dependence on the microsomal triglyceride transfer protein to independence
- PMID: 9843958
- PMCID: PMC24518
- DOI: 10.1073/pnas.95.25.14733
Apoprotein B100 has a prolonged interaction with the translocon during which its lipidation and translocation change from dependence on the microsomal triglyceride transfer protein to independence
Abstract
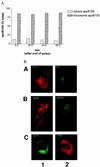
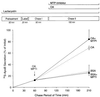
When lipid synthesis is limited in HepG2 cells, apoprotein B100 (apoB100) is not secreted but rapidly degraded by the ubiquitin-proteasome pathway. To investigate apoB100 biosynthesis and secretion further, the physical and functional states of apoB100 destined for either degradation or lipoprotein assembly were studied under conditions in which lipid synthesis, proteasomal activity, and microsomal triglyceride transfer protein (MTP) lipid-transfer activity were varied. Cells were pretreated with a proteasomal inhibitor (which remained with the cells throughout the experiment) and radiolabeled for 15 min. During the chase period, labeled apoB100 remained associated with the microsomes. Furthermore, by crosslinking sec61beta to apoB100, we showed that apoB100 remained close to the translocon at the same time apoB100-ubiquitin conjugates could be detected. When lipid synthesis and lipoprotein assembly/secretion were stimulated by adding oleic acid (OA) to the chase medium, apoB100 was deubiquitinated, and its interaction with sec61beta was disrupted, signifying completion of translocation concomitant with the formation of lipoprotein particles. MTP participates in apoB100 translocation and lipoprotein assembly. In the presence of OA, when MTP lipid-transfer activity was inhibited at the end of pulse labeling, apoB100 secretion was abolished. In contrast, when the labeled apoB100 was allowed to accumulate in the cell for 60 min before adding OA and the inhibitor, apoB100 lipidation and secretion were no longer impaired. Overall, the data imply that during most of its association with the endoplasmic reticulum, apoB100 is close to or within the translocon and is accessible to both the ubiquitin-proteasome and lipoprotein-assembly pathways. Furthermore, MTP lipid-transfer activity seems to be necessary only for early translocation and lipidation events.
Figures


References
-
- Borén J, Graham L, Wettesten M, Scott J, White A, Olofsson S. J Biol Chem. 1992;267:9858–9867. - PubMed
-
- Wu X, Zhou M, Huang L-S, Wetterau J, Ginsberg H N. J Biol Chem. 1996;271:10277–10281. - PubMed
-
- Patel S B, Grundy S M. J Biol Chem. 1996;271:18686–18694. - PubMed
-
- Hussain M M, Bakillah A, Jamil H. Biochemistry. 1997;21:13060–13067. - PubMed
-
- Vance J E, Vance D E. Annu Rev Nutr. 1990;10:337–356. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

