Mature monocytic cells enter tissues and engraft
- PMID: 9843995
- PMCID: PMC24555
- DOI: 10.1073/pnas.95.25.14944
Mature monocytic cells enter tissues and engraft
Abstract
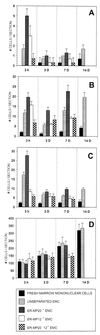
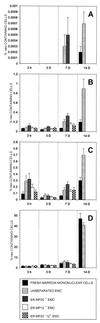
The goal of this study was to identify the circulating cell that is the immediate precursor of tissue macrophages. ROSA 26 marrow mononuclear cells (containing the beta-geo transgene that encodes beta-galactosidase and neomycin resistance activities) were cultured in the presence of macrophage colony-stimulating factor and flt3 Ligand for 6 days to generate monocytic cells at all stages of maturation. Expanded monocyte cells (EMC), the immature (ER-MP12(+)) and more mature (ER-MP20(+)) subpopulations, were transplanted into irradiated B6/129 F2 mice. beta-gal staining of tissue sections from animals 15 min after transplantation demonstrated that the donor cells landed randomly. By 3 h, donor cells in lung and liver were more frequent in animals transplanted with ER-MP20(+) (more mature) EMC than in animals transplanted with unseparated EMC or fresh marrow mononuclear cells, a pattern that persisted at 3 and 7 days. At 3 days, donor cells were found in spleen, liver, lung, and brain (rarely) as clusters as well as individual cells. By 7 and 14 days, the clusters had increased in size, and the cells expressed the macrophage antigen F4/80, suggesting that further replication and differentiation had occurred. PCR for the neogene was used to quantitate the amount of donor DNA in tissues from transplanted animals and confirmed that ER-MP20(+) EMC preferentially engrafted. These data demonstrate that a mature monocytic cell gives rise to tissue macrophages. Because these cells can be expanded and manipulated in vitro, they may be a suitable target population for gene therapy of lysosomal storage diseases.
Figures

References
-
- Neufeld E F, Muenzer J. In: The Metabolic and Molecular Bases of Inherited Disease. Scriver C R, Beaudet A L, Sly W S, Valle D, editors. New York: McGraw–Hill; 1995. pp. 2465–2494.
-
- Grabowski G A, Barton N W, Pastores G, Dambrosia J M, Banerjee T K, McKee M A, Parker C, Schiffmann R, Hill S C, Brady R O. Ann Intern Med. 1995;122:33–39. - PubMed
-
- Kaye E M. Curr Opin Pediatr. 1995;7:650–654. - PubMed
-
- Resnick J M, Krivit W, Snover D C, Kersey J H, Ramsay N K C, Blazar B R, Whitley C B. Bone Marrow Transplant. 1992;10:273–280. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

