Bacteroides fragilis enterotoxin cleaves the zonula adherens protein, E-cadherin
- PMID: 9844001
- PMCID: PMC24561
- DOI: 10.1073/pnas.95.25.14979
Bacteroides fragilis enterotoxin cleaves the zonula adherens protein, E-cadherin
Abstract
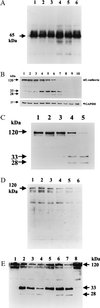
Strains of Bacteroides fragilis associated with diarrheal disease (enterotoxigenic B. fragilis) produce a 20-kDa zinc-dependent metalloprotease toxin (B. fragilis enterotoxin; BFT) that reversibly stimulates chloride secretion and alters tight junctional function in polarized intestinal epithelial cells. BFT alters cellular morphology and physiology most potently and rapidly when placed on the basolateral membrane of epithelial cells, suggesting that the cellular substrate for BFT may be present on this membrane. Herein, we demonstrate that BFT specifically cleaves within 1 min the extracellular domain of the zonula adherens protein, E-cadherin. Cleavage of E-cadherin by BFT is ATP-independent and essential to the morphologic and physiologic activity of BFT. However, the morphologic changes occurring in response to BFT are dependent on target-cell ATP. E-cadherin is shown here to be a cellular substrate for a bacterial toxin and represents the identification of a mechanism of action, cell-surface proteolytic activity, for a bacterial toxin.
Figures



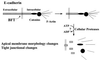
References
-
- Polk F B, Kasper D L. Ann Intern Med. 1996;86:569–571. - PubMed
-
- Tzianabos A O, Onderdonk A B, Rosner B, Cisneros R L, Kasper D L. Science. 1993;262:416–419. - PubMed
-
- Mundy L M, Sears C L. Clin Infect Dis. 1996;23:269–276. - PubMed
-
- Sears, C. L., Myers, L. L., Lazenby, A. & Van Tassell, R. L. (1995) Clin. Infect. Dis. 20, Suppl. 2, S142–S148. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

