Arsenic enhancement of skin neoplasia by chronic stimulation of growth factors
- PMID: 9846968
- PMCID: PMC1866326
- DOI: 10.1016/S0002-9440(10)65692-1
Arsenic enhancement of skin neoplasia by chronic stimulation of growth factors
Abstract
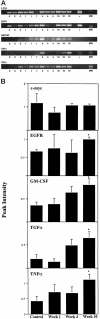
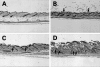
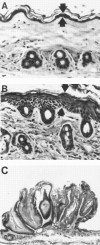
Although numerous epidemiological studies have shown that inorganic arsenicals cause skin cancers and hyperkeratoses in humans, there are currently no established mechanisms for their action or animal models. Previous studies in our laboratory using primary human keratinocyte cultures demonstrated that micromolar concentrations of inorganic arsenite increased cell proliferation via the production of keratinocyte-derived growth factors. As recent reports demonstrate that overexpression of keratinocyte-derived growth factors, such as transforming growth factor (TGF)-alpha, promote the formation of skin tumors, we hypothesized that similar events may be responsible for those associated with arsenic skin diseases. Thus, the influence of arsenic in humans with arsenic skin disease and on mouse skin tumor development in transgenic mice was studied. After low-dose application of tetradecanoyl phorbol acetate (TPA), a marked increase in the number of skin papillomas occurred in Tg.AC mice, which carry the v-Ha-ras oncogene, that received arsenic in the drinking water as compared with control drinking water, whereas no papillomas developed in arsenic-treated transgenic mice that did not receive TPA or arsenic/TPA-treated wild-type FVB/N mice. Consistent with earlier in vitro findings, increases in granulocyte/macrophage colony-stimulating factor (GM-CSF) and TGF-alpha mRNA transcripts were found in the epidermis at clinically normal sites within 10 weeks after arsenic treatment. Immunohistochemical staining localized TGF-alpha overexpression to the hair follicles. Injection of neutralizing antibodies to GM-CSF after TPA application reduced the number of papillomas in Tg.AC mice. Analysis of gene expression in samples of skin lesions obtained from humans chronically exposed to arsenic via their drinking water also showed similar alterations in growth factor expression. Although confirmation will be required in nontransgenic mice, these results suggest that arsenic enhances development of skin neoplasias via the chronic stimulation of keratinocyte-derived growth factors and may be a rare example of a chemical carcinogen that acts as a co-promoter.
Figures





References
-
- US Environmental Protection Agency: Special Report on Ingested Inorganic Arsenic: Skin Cancer and Nutritional Essentiality. Risk Assessment Forum. Washington, DC, US Environmental Protection Agency, 1987
-
- Nriagu JO: Arsenic in the Environment. II. Human Health and Ecosystem Effects. 1994. Wiley and Sons, New York
-
- Chiou HY, Hsueh YM, Liaw KF, Horng SF, Chiang MH, Pu YS, Lin JS, Huang CH, Chen CJ: Incidence of internal cancers and ingested inorganic arsenic: a 7-year follow-up study in Taiwan. Cancer Res 1995, 55:1296-1300 - PubMed
-
- Maloney ME: Arsenic in dermatology. Dermatol Surg 1996, 22:301-304 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

