Acute effects of inhaled urban particles and ozone: lung morphology, macrophage activity, and plasma endothelin-1
- PMID: 9846977
- PMCID: PMC1866316
- DOI: 10.1016/S0002-9440(10)65701-X
Acute effects of inhaled urban particles and ozone: lung morphology, macrophage activity, and plasma endothelin-1
Abstract
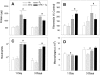
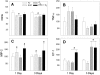
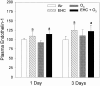
We studied acute responses of rat lungs to inhalation of urban particulate matter and ozone. Exposure to particles (40 mg/m3 for 4 hours; mass median aerodynamic diameter, 4 to 5 microm; Ottawa urban dust, EHC-93), followed by 20 hours in clean air, did not result in acute lung injury. Nevertheless, inhalation of particles resulted in decreased production of nitric oxide (nitrite) and elevated secretion of macrophage inflammatory protein-2 from lung lavage cells. Inhalation of ozone (0.8 parts per million for 4 hours) resulted in increased neutrophils and protein in lung lavage fluid. Ozone alone also decreased phagocytosis and nitric oxide production and stimulated endothelin-1 secretion by lung lavage cells but did not modify secretion of macrophage inflammatory protein-2. Co-exposure to particles potentiated the ozone-induced septal cellularity in the central acinus but without measurable exacerbation of the ozone-related alveolar neutrophilia and permeability to protein detected by lung lavage. The enhanced septal thickening was associated with elevated production of both macrophage inflammatory protein-2 and endothelin-1 by lung lavage cells. Interestingly, inhalation of urban particulate matter increased the plasma levels of endothelin-1, but this response was not influenced by the synergistic effects of ozone and particles on centriacinar septal tissue changes. This suggests an impact of the distally distributed particulate dose on capillary endothelial production or filtration of the vasoconstrictor. Overall, equivalent patterns of effects were observed after a single exposure or three consecutive daily exposures to the pollutants. The experimental data are consistent with epidemiological evidence for acute pulmonary effects of ozone and respirable particulate matter and suggest a possible mechanism whereby cardiovascular effects may be induced by particle exposure. In a broad sense, acute biological effects of respirable particulate matter from ambient air appear related to paracrine/endocrine disruption mechanisms.
Figures



References
-
- Bates DV, Sizto R: Relationships between air pollution levels and hospital admissions in Southern Ontario. Can J Public Health 1983, 74:117-122 - PubMed
-
- Dockery DW, Pope CA, Xu X, Spengler JD, Ware JH, Fay ME, Ferris BG, Speizer FE: An association between air pollution and mortality in six U.S. cities. N Engl J Med 1993, 329:1753-1759 - PubMed
-
- Burnett RT, Dales RE, Raizenne ME, Krewski D, Summers PW, Roberts GR, Raad-Young M, Dann T, Brook J: Effects of low ambient levels of ozone and sulfates on the frequency of respiratory admissions to Ontario hospitals. Environ Res 1994, 65:172-194 - PubMed
-
- Burnett RT, Dales RE, Krewski D, Vincent R, Dann T, Brook JR: Associations between ambient particulate sulfate and admissions to Ontario hospitals for cardiac and respiratory diseases. Am J Epidemiol 1995, 142:15-22 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

