Spermine is a salicylate-independent endogenous inducer for both tobacco acidic pathogenesis-related proteins and resistance against tobacco mosaic virus infection
- PMID: 9847095
- PMCID: PMC34737
- DOI: 10.1104/pp.118.4.1213
Spermine is a salicylate-independent endogenous inducer for both tobacco acidic pathogenesis-related proteins and resistance against tobacco mosaic virus infection
Abstract
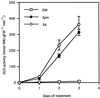
Intercellular spaces are often the first sites invaded by pathogens. In the spaces of tobacco mosaic virus (TMV)-infected and necrotic lesion-forming tobacco (Nicotiana tabacum L.) leaves, we found that an inducer for acidic pathogenesis-related (PR) proteins was accumulated. The induction activity was recovered in gel-filtrated fractions of low molecular mass with a basic nature, into which authentic spermine (Spm) was eluted. We quantified polyamines in the intercellular spaces of the necrotic lesion-forming leaves and found 20-fold higher levels of free Spm than in healthy leaves. Among several polyamines tested, exogenously supplied Spm induced acidic PR-1 gene expression. Immunoblot analysis showed that Spm treatment increased not only acidic PR-1 but also acidic PR-2, PR-3, and PR-5 protein accumulation. Treatment of healthy tobacco leaves with salicylic acid (SA) caused no significant increase in the level of endogenous Spm, and Spm did not increase the level of endogenous SA, suggesting that induction of acidic PR proteins by Spm is independent of SA. The size of TMV-induced local lesions was reduced by Spm treatment. These results indicate that Spm accumulates outside of cells after lesion formation and induces both acidic PR proteins and resistance against TMV via a SA-independent signaling pathway.
Figures










References
-
- Bertossi F, Bagni N, Moruzzi G, Caldarera CM. Spermine as a new growth-promoting substance for Helianthus tuberosus (Jerusalem artichoke) in vitro. Experientia. 1965;21:80–81.
-
- Davis BJ. Method and application to human serum proteins. Ann NY Acad Sci. 1964;121:404–427. - PubMed
-
- De Laat AMM, Van Loon LC. The relationship between stimulated ethylene production and symptom expression in virus-infected tobacco leaves. Physiol Plant Pathol. 1983;22:261–273.
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

