Differential expression and internal feedback regulation of 1-aminocyclopropane-1-carboxylate synthase, 1-aminocyclopropane-1-carboxylate oxidase, and ethylene receptor genes in tomato fruit during development and ripening
- PMID: 9847103
- PMCID: PMC34745
- DOI: 10.1104/pp.118.4.1295
Differential expression and internal feedback regulation of 1-aminocyclopropane-1-carboxylate synthase, 1-aminocyclopropane-1-carboxylate oxidase, and ethylene receptor genes in tomato fruit during development and ripening
Abstract
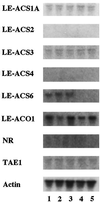
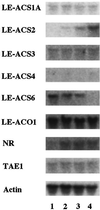
We investigated the feedback regulation of ethylene biosynthesis in tomato (Lycopersicon esculentum) fruit with respect to the transition from system 1 to system 2 ethylene production. The abundance of LE-ACS2, LE-ACS4, and NR mRNAs increased in the ripening fruit concomitant with a burst in ethylene production. These increases in mRNAs with ripening were prevented to a large extent by treatment with 1-methylcyclopropene (MCP), an ethylene action inhibitor. Transcripts for the LE-ACS6 gene, which accumulated in preclimacteric fruit but not in untreated ripening fruit, did accumulate in ripening fruit treated with MCP. Treatment of young fruit with propylene prevented the accumulation of transcripts for this gene. LE-ACS1A, LE-ACS3, and TAE1 genes were expressed constitutively in the fruit throughout development and ripening irrespective of whether the fruit was treated with MCP or propylene. The transcripts for LE-ACO1 and LE-ACO4 genes already existed in preclimacteric fruit and increased greatly when ripening commenced. These increases in LE-ACO mRNA with ripening were also prevented by treatment with MCP. The results suggest that in tomato fruit the preclimacteric system 1 ethylene is possibly mediated via constitutively expressed LE-ACS1A and LE-ACS3 and negatively feedback-regulated LE-ACS6 genes with preexisting LE-ACO1 and LE-ACO4 mRNAs. At the onset of the climacteric stage, it shifts to system 2 ethylene, with a large accumulation of LE-ACS2, LE-ACS4, LE-ACO1, and LE-ACO4 mRNAs as a result of a positive feedback regulation. This transition from system 1 to system 2 ethylene production might be related to the accumulated level of NR mRNA.
Figures




References
-
- Akamine EK, Goo T. Respiration and ethylene production in mammee apple (Mammea americana L.) J Am Soc Hortic Sci. 1978;103:308–310.
-
- Barry CS, Blume B, Bouzayen M, Cooper W, Hamilton AJ, Grierson D. Differential expression of the 1aminocyclopropane-1-carboxylate oxidase gene family of tomato. Plant J. 1996;9:525–535. - PubMed
-
- Biale JB, Young RE. Respiration and ripening in fruits—retrospect and prospect. In: Friend J, Rhodes MJC, editors. Recent Advances in the Biochemistry of Fruits and Vegetables. London: Academic Press; 1981. pp. 1–39.
-
- Chang C, Kwok SF, Bleeker AB, Meyerowitz EM. Arabidopsis ethylene-response gene ETR1: similarity of product to two-component regulators. Science. 1993;262:539–544. - PubMed
-
- Fluhr R, Mattoo AK. Ethylene—biosynthesis and perception. CRC Crit Rev Plant Sci. 1996;15:479–523.
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

