Comparison of the ability of partially N-acetylated chitosans and chitooligosaccharides to elicit resistance reactions in wheat leaves
- PMID: 9847109
- PMCID: PMC34751
- DOI: 10.1104/pp.118.4.1353
Comparison of the ability of partially N-acetylated chitosans and chitooligosaccharides to elicit resistance reactions in wheat leaves
Abstract
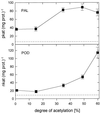
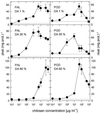
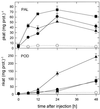
Chitin, a linear polysaccharide composed of (1-->4)-linked 2-acetamido-2-deoxy-beta-D-glucopyranose (GlcNAc) residues, and chitosan, the fully or partially N-acetylated, water-soluble derivative of chitin composed of (1-->4)-linked GlcNAc and 2-amino-2-deoxy-beta-D-glucopyranose (GlcN), have been proposed as elicitors of defense reactions in higher plants. We tested and compared the ability of purified (1-->4)-linked oligomers of GlcNAc (tetramer to decamer) and of GlcN (pentamer and heptamer) and partially N-acetylated chitosans with degrees of acetylation (DA) of 1%, 15%, 35%, 49%, and 60% and average degrees of polymerization between 540 and 1100 to elicit phenylalanine ammonia-lyase (PAL) and peroxidase (POD) activities, lignin deposition, and microscopically and macroscopically visible necroses when injected into the intercellular spaces of healthy, nonwounded wheat (Triticum aestivum L.) leaves. Purified oligomers of (1-->4)-linked GlcN were not active as elicitors, whereas purified oligomers of (1-->4)-linked GlcNAc with a degree of polymerization >/= 7 strongly elicited POD activities but not PAL activities. Partially N-acetylated, polymeric chitosans elicited both PAL and POD activities, and maximum elicitation was observed with chitosans of intermediate DAs. All chitosans but not the chitin oligomers induced the deposition of lignin, the appearance of necrotic cells exhibiting yellow autofluorescence under ultraviolet light, and macroscopically visible necroses; those with intermediate DAs were most active. These results suggest that different mechanisms are involved in the elicitation of POD activities by GlcNAc oligomers, and of PAL and POD activities by partially N-acetylated chitosan polymers and that both enzymes have to be activated for lignin biosynthesis and ensuing necrosis to occur.
Figures




References
-
- Albersheim P, Anderson-Prouty AJ. Carbohydrates, proteins, cell surfaces, and biochemistry of pathogenesis. Annu Rev Plant Physiol. 1975;26:31–52.
-
- Anthonsen MW, Vårum KM, Smidsrød O. Solution properties of chitosans: conformation and chain stiffness of chitosans with different degrees of N-acetylation. Carbohydr Polym. 1993;22:193–201.
-
- Araki Y, Ito E. A pathway of chitosan formation in M. rouxii. Eur J Biochem. 1975;55:71–78. - PubMed
-
- Arlorio M, Ludwig A, Boller T, Bonfante P. Inhibition of fungal growth by plant chitinases and β-1,3-glucanases. A morphological study. Protoplasma. 1992;171:34–43.
-
- Barber MS, Bertram RE, Ride JP. Chitin oligosaccharides elicit lignification in wounded wheat leaves. Physiol Mol Plant Pathol. 1989;34:3–12.
LinkOut - more resources
Full Text Sources
Other Literature Sources

