High-molecular-weight FK506-binding proteins are components of heat-shock protein 90 heterocomplexes in wheat germ lysate
- PMID: 9847114
- PMCID: PMC34756
- DOI: 10.1104/pp.118.4.1395
High-molecular-weight FK506-binding proteins are components of heat-shock protein 90 heterocomplexes in wheat germ lysate
Abstract
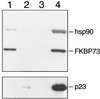
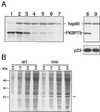
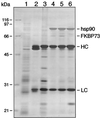
In animal cell lysates the multiprotein heat-shock protein 90 (hsp90)-based chaperone complexes consist of hsp70, hsp40, and p60. These complexes act to convert steroid hormone receptors to their steroid-binding state by assembling them into heterocomplexes with hsp90, p23, and one of several immunophilins. Wheat germ lysate also contains a hsp90-based chaperone system that can assemble the glucocorticoid receptor into a functional heterocomplex with hsp90. However, only two components of the heterocomplex-assembly system, hsp90 and hsp70, have thus far been identified. Recently, purified mammalian p23 preadsorbed with JJ3 antibody-protein A-Sepharose pellets was used to isolate a mammalian p23-wheat hsp90 heterocomplex from wheat germ lysate (J.K. Owens-Grillo, L.F. Stancato, K. Hoffmann, W.B. Pratt, and P. Krishna [1996] Biochemistry 35: 15249-15255). This heterocomplex was found to contain an immunophilin(s) of the FK506-binding class, as judged by binding of the radiolabeled immunosuppressant drug [3H]FK506 to the immune pellets in a specific manner. In the present study we identified the immunophilin components of this heterocomplex as FKBP73 and FKBP77, the two recently described high-molecular-weight FKBPs of wheat. In addition, we present evidence that the two FKBPs bind hsp90 via tetratricopeptide repeat domains. Our results demonstrate that binding of immunophilins to hsp90 via tetratricopeptide repeat domains is a conserved protein interaction in plants. Conservation of this protein-to-protein interaction in both plant and animal cells suggests that it is important for the biological action of the high-molecular-weight immunophilins.
Figures


Similar articles
-
Binding of immunophilins to the 90 kDa heat shock protein (hsp90) via a tetratricopeptide repeat domain is a conserved protein interaction in plants.Biochemistry. 1996 Dec 3;35(48):15249-55. doi: 10.1021/bi9615349. Biochemistry. 1996. PMID: 8952474
-
The cyclosporin A-binding immunophilin CyP-40 and the FK506-binding immunophilin hsp56 bind to a common site on hsp90 and exist in independent cytosolic heterocomplexes with the untransformed glucocorticoid receptor.J Biol Chem. 1995 Sep 1;270(35):20479-84. doi: 10.1074/jbc.270.35.20479. J Biol Chem. 1995. PMID: 7657624
-
All of the protein interactions that link steroid receptor.hsp90.immunophilin heterocomplexes to cytoplasmic dynein are common to plant and animal cells.Biochemistry. 2002 Apr 30;41(17):5581-7. doi: 10.1021/bi020073q. Biochemistry. 2002. PMID: 11969419
-
A model for the cytoplasmic trafficking of signalling proteins involving the hsp90-binding immunophilins and p50cdc37.Cell Signal. 1999 Dec;11(12):839-51. doi: 10.1016/s0898-6568(99)00064-9. Cell Signal. 1999. PMID: 10659992 Review.
-
Regulation of signaling protein function and trafficking by the hsp90/hsp70-based chaperone machinery.Exp Biol Med (Maywood). 2003 Feb;228(2):111-33. doi: 10.1177/153537020322800201. Exp Biol Med (Maywood). 2003. PMID: 12563018 Review.
Cited by
-
The Hsp90 family of proteins in Arabidopsis thaliana.Cell Stress Chaperones. 2001 Jul;6(3):238-46. doi: 10.1379/1466-1268(2001)006<0238:thfopi>2.0.co;2. Cell Stress Chaperones. 2001. PMID: 11599565 Free PMC article.
-
Arabidopsis immunophilin-like TWD1 functionally interacts with vacuolar ABC transporters.Mol Biol Cell. 2004 Jul;15(7):3393-405. doi: 10.1091/mbc.e03-11-0831. Epub 2004 May 7. Mol Biol Cell. 2004. PMID: 15133126 Free PMC article.
-
Characterization of plant p23-like proteins for their co-chaperone activities.Cell Stress Chaperones. 2010 Sep;15(5):703-15. doi: 10.1007/s12192-010-0182-1. Epub 2010 Mar 28. Cell Stress Chaperones. 2010. PMID: 20349287 Free PMC article.
-
Deletion of the C-terminal 138 amino acids of the wheat FKBP73 abrogates calmodulin binding, dimerization and male fertility in transgenic rice.Plant Mol Biol. 2002 Mar;48(4):369-81. doi: 10.1023/a:1014023329807. Plant Mol Biol. 2002. PMID: 11905964
-
Orthologs in Arabidopsis thaliana of the Hsp70 interacting protein Hip.Cell Stress Chaperones. 2001 Jul;6(3):247-55. doi: 10.1379/1466-1268(2001)006<0247:oiatot>2.0.co;2. Cell Stress Chaperones. 2001. PMID: 11599566 Free PMC article.
References
-
- Blecher O, Erel N, Callebaut I, Aviezer K, Breiman A. A novel plant peptidylprolyl-cis-trans-isomerase (PPIase): cDNA cloning, structural analysis, enzymatic activity and expression. Plant Mol Biol. 1996;32:493–504. - PubMed
-
- Bose S, Weikl T, Bugl H, Buchner J. Chaperone function of hsp90-associated proteins. Science. 1996;274:1715–1717. - PubMed
-
- Boston RS, Viitanen PV, Vierling E. Molecular chaperones and protein folding in plants. Plant Mol Biol. 1996;32:191–222. - PubMed
-
- Chen M-S, Silverstein AM, Pratt WB, Chinkers M. The tetratricopeptide repeat domain of protein phosphatase 5 mediates binding to glucocorticoid receptor heterocomplexes and acts as a dominant negative mutant. J Biol Chem. 1996;271:32315–32320. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

